Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images
- PMID: 20367663
- PMCID: PMC3823118
- DOI: 10.1111/j.1582-4934.2010.01060.x
Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images
Abstract
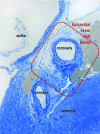
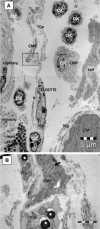
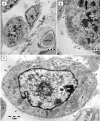
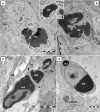
A highly heterogeneous population of stem and progenitor cells has been described by light immunohistochemistry in the mammalian adult heart, but the ultrastructural identity of cardiac stem cells remains unknown. Using electron microscopy, we demonstrate the presence of cells with stem features in the adult mouse heart. These putative cardiac stem cells are small (6-10 microm), round cells, with an irregular shaped nucleus, large nucleolus, few endoplasmic reticulum cisternae and mitochondria, but numerous ribosomes. Stem cells located in the epicardial stem cell niche undergo mitosis and apoptosis. Cells with intermediate features between stem cells and cardiomyocyte progenitors have also been seen. Moreover, electron microscopy showed that cardiomyocyte progenitors were added to the peripheral working cardiomyocytes. Telocytes make a supportive interstitial network for stem cells and progenitors in the stem cell niche. This study enhances the hypothesis of a unique type of cardiac stem cell and progenitors in different stages of differentiation. In our opinion, stem cells, cardiomyocyte progenitors and telocytes sustain a continuous cardiac renewal process in the adult mammalian heart.
Figures

Similar articles
-
Telocytes in human epicardium.J Cell Mol Med. 2010 Aug;14(8):2085-93. doi: 10.1111/j.1582-4934.2010.01129.x. Epub 2010 Jul 13. J Cell Mol Med. 2010. PMID: 20629996 Free PMC article.
-
Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches.J Cell Mol Med. 2009 May;13(5):866-86. doi: 10.1111/j.1582-4934.2009.00758.x. Epub 2009 Apr 20. J Cell Mol Med. 2009. PMID: 19382895 Free PMC article.
-
Telocytes as supporting cells for myocardial tissue organization in developing and adult heart.J Cell Mol Med. 2010 Oct;14(10):2531-8. doi: 10.1111/j.1582-4934.2010.01119.x. J Cell Mol Med. 2010. PMID: 20977627 Free PMC article.
-
A Tale of Two Cells: Telocyte and Stem Cell Unique Relationship.Adv Exp Med Biol. 2016;913:359-376. doi: 10.1007/978-981-10-1061-3_23. Adv Exp Med Biol. 2016. PMID: 27796899 Review.
-
Telocytes in Cardiac Tissue Architecture and Development.Adv Exp Med Biol. 2016;913:127-137. doi: 10.1007/978-981-10-1061-3_8. Adv Exp Med Biol. 2016. PMID: 27796884 Review.
Cited by
-
Telocytes and putative stem cells in ageing human heart.J Cell Mol Med. 2015 Jan;19(1):31-45. doi: 10.1111/jcmm.12509. Epub 2014 Dec 25. J Cell Mol Med. 2015. PMID: 25545142 Free PMC article.
-
Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis.J Cell Mol Med. 2015 Jul;19(7):1720-8. doi: 10.1111/jcmm.12548. Epub 2015 Mar 6. J Cell Mol Med. 2015. PMID: 25753567 Free PMC article.
-
Telocytes in human skin--are they involved in skin regeneration?J Cell Mol Med. 2012 Jul;16(7):1405-20. doi: 10.1111/j.1582-4934.2012.01580.x. J Cell Mol Med. 2012. PMID: 22500885 Free PMC article.
-
Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis.J Cell Mol Med. 2013 Apr;17(4):482-96. doi: 10.1111/jcmm.12028. Epub 2013 Feb 27. J Cell Mol Med. 2013. PMID: 23444845 Free PMC article.
-
Tissue engineering and regenerative medicine -where do we stand?J Cell Mol Med. 2012 Jun;16(6):1157-65. doi: 10.1111/j.1582-4934.2012.01564.x. J Cell Mol Med. 2012. PMID: 22436120 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

