The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation
- PMID: 20367757
- PMCID: PMC3510705
- DOI: 10.1111/j.1471-4159.2010.06706.x
The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation
Abstract
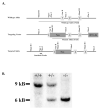
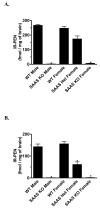
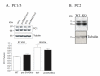
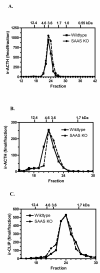
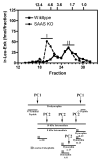
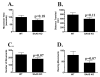
Mice with a targeted mutation in proSAAS have been generated to investigate whether peptides derived from this precursor could function as an inhibitor of prohormone convertase 1/3 (PC1/3) in vivo as well as to determine any alternate roles for proSAAS in nervous and endocrine tissues. Fetal mice lacking proSAAS exhibit complete, adult-like processing of prodynorphin in the prenatal brain instead of the incomplete processing seen in the brains of wild-type fetal mice where inhibitory proSAAS intermediates are transiently accumulated. This study provides evidence that proSAAS is directly involved in the prenatal regulation of neuropeptide processing in vivo. However, adult mice lacking proSAAS have normal levels of all peptides detected using a peptidomics approach, suggesting that PC1/3 activity is not affected by the absence of proSAAS in adult mice. ProSAAS knockout mice exhibit decreased locomotion and a male-specific 10-15% decrease in body weight, but maintain normal fasting blood glucose levels and are able to efficiently clear glucose from the blood in response to a glucose challenge. This work suggests that proSAAS-derived peptides can inhibit PC1/3 in embryonic brain, but in the adult brain proSAAS peptides may function as neuropeptides that regulate body weight and potentially other behaviors.
Figures

Similar articles
-
Tissue distribution and processing of proSAAS by proprotein convertases.J Neurochem. 2001 Mar;76(6):1833-41. doi: 10.1046/j.1471-4159.2001.00165.x. J Neurochem. 2001. PMID: 11259501
-
Obesity and diabetes in transgenic mice expressing proSAAS.J Endocrinol. 2004 Mar;180(3):357-68. doi: 10.1677/joe.0.1800357. J Endocrinol. 2004. PMID: 15012590
-
Neuropeptide processing profile in mice lacking prohormone convertase-1.Biochemistry. 2005 Mar 29;44(12):4939-48. doi: 10.1021/bi047852m. Biochemistry. 2005. PMID: 15779921
-
On the tissue-specific processing of procholecystokinin in the brain and gut--a short review.J Physiol Pharmacol. 2003 Dec;54 Suppl 4:73-9. J Physiol Pharmacol. 2003. PMID: 15075450 Review.
-
Neuropeptide processing and its impact on melanocortin pathways.Endocrinology. 2007 Sep;148(9):4201-7. doi: 10.1210/en.2006-1686. Epub 2007 Jun 21. Endocrinology. 2007. PMID: 17584964 Review.
Cited by
-
A novel function for proSAAS as an amyloid anti-aggregant in Alzheimer's disease.J Neurochem. 2014 Feb;128(3):419-30. doi: 10.1111/jnc.12454. Epub 2013 Oct 24. J Neurochem. 2014. PMID: 24102330 Free PMC article.
-
The neural chaperone proSAAS blocks α-synuclein fibrillation and neurotoxicity.Proc Natl Acad Sci U S A. 2016 Aug 9;113(32):E4708-15. doi: 10.1073/pnas.1601091113. Epub 2016 Jul 25. Proc Natl Acad Sci U S A. 2016. PMID: 27457957 Free PMC article.
-
ProSAAS-derived peptides are colocalized with neuropeptide Y and function as neuropeptides in the regulation of food intake.PLoS One. 2011;6(12):e28152. doi: 10.1371/journal.pone.0028152. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164236 Free PMC article.
-
The extended granin family: structure, function, and biomedical implications.Endocr Rev. 2011 Dec;32(6):755-97. doi: 10.1210/er.2010-0027. Epub 2011 Aug 23. Endocr Rev. 2011. PMID: 21862681 Free PMC article. Review.
-
PCSK1 Variants and Human Obesity.Prog Mol Biol Transl Sci. 2016;140:47-74. doi: 10.1016/bs.pmbts.2015.12.001. Epub 2016 Jan 29. Prog Mol Biol Transl Sci. 2016. PMID: 27288825 Free PMC article. Review.
References
-
- Apletalina E, Appel J, Lamango NS, Houghten RA, Lindberg I. Identification of inhibitors of prohormone convertases 1 and 2 using a peptide combinatorial library. Journal of Biological Chemistry. 1998;273:26589–26595. - PubMed
-
- Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chretien M, Seidah NG. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. The Journal of biological chemistry. 2001;276:32720–32728. - PubMed
-
- Bennet HPL. Glycosylation, phosphorylation, and sulfation of peptide hormones and their precursors: Peptide Biosynthesis and Processing. CRC Press; Boca Raton, FL: 1991.
-
- Berman Y, Mzhavia N, Polonskaia A, Devi LA. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. Journal of Biological Chemistry. 2001;276:1466–1473. - PubMed
-
- Cameron A, Fortenberry Y, Lindberg I. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1/3. FEBS Letters. 2000;473:135–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

