Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo
- PMID: 20368175
- PMCID: PMC2852889
- DOI: 10.1126/scitranslmed.3000111
Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo
Erratum in
- Sci Transl Med. 2009 Nov 4;1(5):5ra13
Abstract
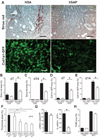
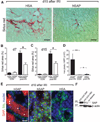
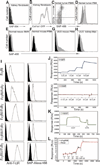
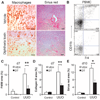
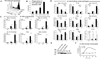
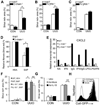
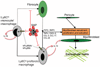
New therapies that target chronic inflammation with fibrosis are urgently required. Increasing evidence points to innate activation of inflammatory cells in driving chronic organ fibrosis. Serum amyloid P is a naturally circulating soluble pattern recognition receptor, a member of the family of pentraxin proteins. It links danger-associated molecular pattern recognition to Fc gamma receptor-mediated phagocytosis. Here we show that fibrosis progression in the mouse kidney is significantly inhibited by therapeutic administration of human serum amyloid P, regulated by activating Fc gamma receptors, and dependent on inflammatory monocytes and macrophages, but not fibrocytes. Human serum amyloid P-mediated inhibition of mouse kidney fibrosis correlated with specific binding of human serum amyloid P to cell debris and with subsequent suppression of inflammatory monocytes and kidney macrophages in vitro and in vivo, and was dependent on regulated binding to activating Fc gamma receptors and interleukin-10 expression. These studies uncover previously unidentified roles for Fc gamma receptors in sterile inflammation and highlight serum amyloid P as a potential antifibrotic therapy through local generation of interleukin-10.
Figures
References
-
- Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat. Immunol. 2006;7:1237–1242. - PubMed
-
- Eddy AA. Progression in chronic kidney disease. Adv. Chronic Kidney Dis. 2005;12:353–365. - PubMed
-
- Lupher ML, Jr, Gallatin WM. Regulation of fibrosis by the immune system. Adv. Immunol. 2006;89:245–288. - PubMed
-
- Duffield JS. The inflammatory macrophage: A story of Jekyll and Hyde. Clin. Sci. (Lond.) 2003;104:27–38. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

