Follistatin gene delivery enhances muscle growth and strength in nonhuman primates
- PMID: 20368179
- PMCID: PMC2852878
- DOI: 10.1126/scitranslmed.3000112
Follistatin gene delivery enhances muscle growth and strength in nonhuman primates
Abstract
Antagonists of myostatin, a blood-borne negative regulator of muscle growth produced in muscle cells, have shown considerable promise for enhancing muscle mass and strength in rodent studies and could serve as potential therapeutic agents for human muscle diseases. One of the most potent of these agents, follistatin, is both safe and effective in mice, but similar tests have not been performed in nonhuman primates. To assess this important criterion for clinical translation, we tested an alternatively spliced form of human follistatin that affects skeletal muscle but that has only minimal effects on nonmuscle cells. When injected into the quadriceps of cynomolgus macaque monkeys, a follistatin isoform expressed from an adeno-associated virus serotype 1 vector, AAV1-FS344, induced pronounced and durable increases in muscle size and strength. Long-term expression of the transgene did not produce any abnormal changes in the morphology or function of key organs, indicating the safety of gene delivery by intramuscular injection of an AAV1 vector. Our results, together with the findings in mice, suggest that therapy with AAV1-FS344 may improve muscle mass and function in patients with certain degenerative muscle disorders.
Conflict of interest statement
Competing interests: J.R.M., B.K.K., and their institution (Nationwide Children’s Hospital) have filed a provisional patent on gene delivery of myostatin inhibitors and follistatin use.
Figures
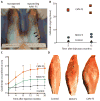
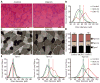
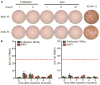

Comment in
-
Regulatory considerations for translating gene therapy: a European Union perspective.Sci Transl Med. 2009 Nov 11;1(6):6ps6. doi: 10.1126/scitranslmed.3000420. Sci Transl Med. 2009. PMID: 20368176
References
-
- Sjöqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–1882. - PubMed
-
- Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, King W, Signore L, Pandya S, Florence J. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. - PubMed
-
- Moxley RT, III, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, Baumbach L, McDonald C, Sussman M, Wade C. Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society, Practice parameter: Corticosteroid treatment of Duchenne dystrophy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. - PubMed
-
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;23:CD003725. - PubMed
-
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Viollet L, Walker CM, Sahenk Z, Clark KR. Limb-girdle muscular dystrophy type 2D gene therapy restores a-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

