In situ regulation of DC subsets and T cells mediates tumor regression in mice
- PMID: 20368186
- PMCID: PMC2872791
- DOI: 10.1126/scitranslmed.3000359
In situ regulation of DC subsets and T cells mediates tumor regression in mice
Abstract
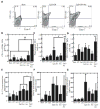
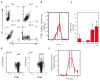
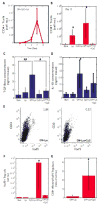
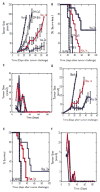
Vaccines are largely ineffective for patients with established cancer, as advanced disease requires potent and sustained activation of CD8(+) cytotoxic T lymphocytes (CTLs) to kill tumor cells and clear the disease. Recent studies have found that subsets of dendritic cells (DCs) specialize in antigen cross-presentation and in the production of cytokines, which regulate both CTLs and T regulatory (Treg) cells that shut down effector T cell responses. Here, we addressed the hypothesis that coordinated regulation of a DC network, and plasmacytoid DCs (pDCs) and CD8(+) DCs in particular, could enhance host immunity in mice. We used functionalized biomaterials incorporating various combinations of an inflammatory cytokine, immune danger signal, and tumor lysates to control the activation and localization of host DC populations in situ. The numbers of pDCs and CD8(+) DCs, and the endogenous production of interleukin-12, all correlated strongly with the magnitude of protective antitumor immunity and the generation of potent CD8(+) CTLs. Vaccination by this method maintained local and systemic CTL responses for extended periods while inhibiting FoxP3 Treg activity during antigen clearance, resulting in complete regression of distant and established melanoma tumors. The efficacy of this vaccine as a monotherapy against large invasive tumors may be a result of the local activity of pDCs and CD8(+) DCs induced by persistent danger and antigen signaling at the vaccine site. These results indicate that a critical pattern of DC subsets correlates with the evolution of therapeutic antitumor responses and provide a template for future vaccine design.
Conflict of interest statement
Figures



Similar articles
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants.Cancer Res. 2014 Mar 15;74(6):1670-81. doi: 10.1158/0008-5472.CAN-13-0777. Epub 2014 Jan 30. Cancer Res. 2014. PMID: 24480625 Free PMC article.
-
Relationship of vaccine efficacy to the kinetics of DC and T-cell responses induced by PLG-based cancer vaccines.Biomatter. 2011 Jul-Sep;1(1):66-75. doi: 10.4161/biom.1.1.16277. Biomatter. 2011. PMID: 23507728 Free PMC article.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Advances in dendritic cell-based vaccine of cancer.Cancer Biother Radiopharm. 2002 Dec;17(6):601-19. doi: 10.1089/108497802320970217. Cancer Biother Radiopharm. 2002. PMID: 12537664 Review.
Cited by
-
Engineering New Approaches to Cancer Vaccines.Cancer Immunol Res. 2015 Aug;3(8):836-43. doi: 10.1158/2326-6066.CIR-15-0112. Epub 2015 Jul 8. Cancer Immunol Res. 2015. PMID: 26156157 Free PMC article. Review.
-
Treating metastatic cancer with nanotechnology.Nat Rev Cancer. 2011 Dec 23;12(1):39-50. doi: 10.1038/nrc3180. Nat Rev Cancer. 2011. PMID: 22193407 Review.
-
Coronavirus disease 2019: A tissue engineering and regenerative medicine perspective.Stem Cells Transl Med. 2021 Jan;10(1):27-38. doi: 10.1002/sctm.20-0197. Epub 2020 Aug 21. Stem Cells Transl Med. 2021. PMID: 32820868 Free PMC article. Review.
-
Strategies for designing synthetic immune agonists.Immunology. 2016 Aug;148(4):315-25. doi: 10.1111/imm.12622. Epub 2016 Jul 11. Immunology. 2016. PMID: 27213842 Free PMC article. Review.
-
Prediction of individual immune responsiveness to a candidate vaccine by a systems vaccinology approach.J Transl Med. 2014 Jan 15;12:11. doi: 10.1186/1479-5876-12-11. J Transl Med. 2014. PMID: 24428943 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

