Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance
- PMID: 20368189
- PMCID: PMC2856693
- DOI: 10.1126/scitranslmed.3000284
Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance
Abstract
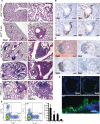
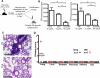
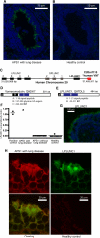
Interstitial lung disease (ILD) is a common manifestation of systemic autoimmunity characterized by progressive inflammation or scarring of the lungs. Patients who develop these complications can exhibit significantly impaired gas exchange that may result in hypoxemia, pulmonary hypertension, and even death. Unfortunately, little is understood about how these diseases arise, including the role of specific defects in immune tolerance. Another key question is whether autoimmune responses targeting the lung parenchyma are critical to ILD pathogenesis, including that of isolated idiopathic forms. We show that a specific defect in central tolerance brought about by mutations in the autoimmune regulator gene (Aire) leads to an autoreactive T cell response to a lung antigen named vomeromodulin and the development of ILD. We found that a human patient and mice with defects in Aire develop similar lung pathology, demonstrating that the AIRE-deficient model of autoimmunity is a suitable translational system in which to unravel fundamental mechanisms of ILD pathogenesis.
Figures



References
-
- Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. - PubMed
-
- Jindal SK, Agarwal R. Autoimmunity and interstitial lung disease. Curr.Opin.Pulm.Med. 2005;11:438–446. - PubMed
-
- Travis WD, Hunninghake G, King TE, Jr, Lynch DA, Colby TV, Galvin JR, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am.J.Respir.Crit.Care Med. 2008;177:1338–1347. - PubMed
-
- American Thoracic Society and European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am.J.Respir.Crit.Care Med. 2002;165:277–304. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- DK59958/DK/NIDDK NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- T32 DK007418/DK/NIDDK NIH HHS/United States
- P01 AI035297/AI/NIAID NIH HHS/United States
- L30 HL098006/HL/NHLBI NIH HHS/United States
- CAPMC/ CIHR/Canada
- K08 DK059958/DK/NIDDK NIH HHS/United States
- R01CA136753A01/CA/NCI NIH HHS/United States
- AI035297/AI/NIAID NIH HHS/United States
- EY016408/EY/NEI NIH HHS/United States
- R01 EY016408/EY/NEI NIH HHS/United States
- K08HL095659/HL/NHLBI NIH HHS/United States
- R01 CA136753/CA/NCI NIH HHS/United States
- K08 HL095659/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

