Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis
- PMID: 20368281
- PMCID: PMC3001131
- DOI: 10.4049/jimmunol.1000217
Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis
Abstract
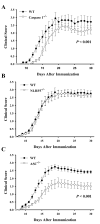
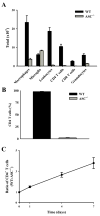
Multiple sclerosis is an autoimmune disease in which self-reactive T cells attack oligodendrocytes that myelinate axons in the CNS. Experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, is dependent on caspase-1; however, the role of Nod-like receptors upstream of caspase-1 is unknown. Danger- and pathogen-associated molecular patterns activate Nod-like receptor 3, which activates caspase-1 through the adaptor protein, apoptosis-associated speck-like protein containing CARD (ASC). We report that the progression of EAE is dependent on ASC and caspase-1 but not Nod-like receptor 3. ASC(-/-) mice were even more protected from the progression of EAE than were caspase-1(-/-) mice, suggesting that an inflammasome-independent function of ASC contributes to the progression of EAE. We found that CD4(+) T cells deficient in ASC exhibited impaired survival; accordingly, ASC(-/-) mice had fewer myelin oligodendrocyte glycoprotein-specific T cells in the draining lymph nodes and CNS.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

Similar articles
-
Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice.J Neuroimmunol. 2005 Mar;160(1-2):110-21. doi: 10.1016/j.jneuroim.2004.11.004. Epub 2004 Dec 30. J Neuroimmunol. 2005. PMID: 15710464
-
T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis.Nat Immunol. 2016 May;17(5):583-92. doi: 10.1038/ni.3389. Epub 2016 Mar 21. Nat Immunol. 2016. PMID: 26998763 Free PMC article.
-
Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4-/- and CD8-/- DBA/1 mice defines qualitative roles of different T cell subsets.J Neuroimmunol. 2003 Aug;141(1-2):10-9. doi: 10.1016/s0165-5728(03)00210-8. J Neuroimmunol. 2003. PMID: 12965249
-
Swift entry of myelin-specific T lymphocytes into the central nervous system in spontaneous autoimmune encephalomyelitis.J Immunol. 2008 Oct 1;181(7):4648-55. doi: 10.4049/jimmunol.181.7.4648. J Immunol. 2008. PMID: 18802067 Free PMC article.
-
Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).Brain Pathol. 2017 Mar;27(2):213-219. doi: 10.1111/bpa.12477. Brain Pathol. 2017. PMID: 27997058 Free PMC article. Review.
Cited by
-
Central and overlapping role of Cathepsin B and inflammasome adaptor ASC in antigen presenting function of human dendritic cells.Hum Immunol. 2012 Sep;73(9):871-8. doi: 10.1016/j.humimm.2012.06.008. Epub 2012 Jun 22. Hum Immunol. 2012. PMID: 22732093 Free PMC article.
-
Type I IFN-mediated regulation of IL-1 production in inflammatory disorders.Cell Mol Life Sci. 2012 Oct;69(20):3395-418. doi: 10.1007/s00018-012-0989-2. Epub 2012 Apr 24. Cell Mol Life Sci. 2012. PMID: 22527721 Free PMC article. Review.
-
Increased interleukin-10 production by ASC-deficient CD4+ T cells impairs bystander T-cell proliferation.Immunology. 2011 Sep;134(1):33-40. doi: 10.1111/j.1365-2567.2011.03462.x. Epub 2011 Jun 30. Immunology. 2011. PMID: 21718313 Free PMC article.
-
A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation.Nat Commun. 2018 May 23;9(1):2036. doi: 10.1038/s41467-018-04376-5. Nat Commun. 2018. PMID: 29789522 Free PMC article.
-
The Effects of IFN-β 1a on the Expression of Inflammasomes and Apoptosis-Associated Speck-Like Proteins in Multiple Sclerosis Patients.Mol Neurobiol. 2017 May;54(4):3031-3037. doi: 10.1007/s12035-016-9864-8. Epub 2016 Mar 31. Mol Neurobiol. 2017. PMID: 27032392 Clinical Trial.
References
-
- Owens T, Tran E, Hassan-Zahraee M, Krakowski M. Immune cell entry to the CNS--a focus for immunoregulation of EAE. Res Immunol. 1998;149:781–789. discussion 844–786, 855–760. - PubMed
-
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. - PubMed
-
- Sutterwala FS, Ogura Y, Zamboni DS, Roy CR, Flavell RA. NALP3: a key player in caspase-1 activation. J Endotoxin Res. 2006;12:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

