Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells
- PMID: 20368348
- PMCID: PMC2876547
- DOI: 10.1128/IAI.01389-09
Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells
Abstract
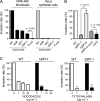
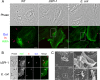
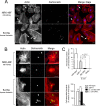
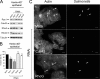
Fibroblasts are ubiquitous cells essential to tissue homeostasis. Despite their nonphagocytic nature, fibroblasts restrain replication of intracellular bacterial pathogens such as Salmonella enterica serovar Typhimurium. The extent to which the entry route of the pathogen determines this intracellular response is unknown. Here, we analyzed S. Typhimurium invasion in fibroblasts obtained from diverse origins, including primary cultures and stable nontransformed cell lines derived from normal tissues. Features distinct to the invasion of epithelial cells were found in all fibroblasts tested. In some fibroblasts, bacteria lacking the type III secretion system encoded in the Salmonella pathogenicity island 1 displayed significant invasion rates and induced the formation of lamellipodia and filopodia at the fibroblast-bacteria contact site. Other bacterial invasion traits observed in fibroblasts were the requirement of phosphatidylinositol 3-kinase, mitogen-activated protein kinase MEK1, and both actin filaments and microtubules. RNA interference studies showed that different Rho family GTPases are targeted by S. Typhimurium to enter into distinct fibroblasts. Rac1 and Cdc42 knockdown affected invasion of normal rat kidney fibroblasts, whereas none of the GTPases tested (Rac1, Cdc42, RhoA, or RhoG) was essential for invasion of immortalized human foreskin fibroblasts. Collectively, these data reveal a marked diversity in the modes used by S. Typhimurium to enter into fibroblasts.
Figures





References
-
- Bakowski, M. A., V. Braun, and J. H. Brumell. 2008. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic 9:2022-2031. - PubMed
-
- Biswas, D., K. Itoh, and C. Sasakawa. 2003. Role of microfilaments and microtubules in the invasion of INT-407 cells by Campylobacter jejuni. Microbiol. Immunol. 47:469-473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

