The Ras effector RASSF2 controls the PAR-4 tumor suppressor
- PMID: 20368356
- PMCID: PMC2876522
- DOI: 10.1128/MCB.00208-09
The Ras effector RASSF2 controls the PAR-4 tumor suppressor
Abstract
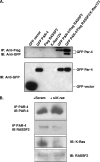
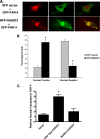
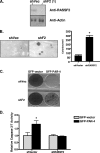
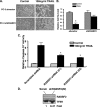
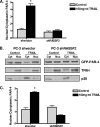
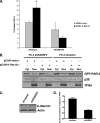
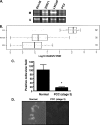
RASSF2 is a novel proapoptotic effector of K-Ras. Inhibition of RASSF2 expression enhances the transforming effects of K-Ras, and epigenetic inactivation of RASSF2 is frequently detected in mutant Ras-containing primary tumors. Thus, RASSF2 is implicated as a tumor suppressor whose inactivation facilitates transformation by disconnecting apoptotic responses from Ras. The mechanism of action of RASSF2 is not known. Here we show that RASSF2 forms a direct and endogenous complex with the prostate apoptosis response protein 4 (PAR-4) tumor suppressor. This interaction is regulated by K-Ras and is essential for the full apoptotic effects of PAR-4. RASSF2 is primarily a nuclear protein, and shuttling of PAR-4 from the cytoplasm to the nucleus is essential for its function. We show that RASSF2 modulates the nuclear translocation of PAR-4 in prostate tumor cells, providing a mechanism for its biological effects. Thus, we identify the first tumor suppressor signaling pathway emanating from RASSF2, we identify a novel mode of action of a RASSF protein, and we provide an explanation for the extraordinarily high frequency of RASSF2 inactivation we have observed in primary prostate tumors.
Figures

Similar articles
-
Ras signaling through RASSF proteins.Semin Cell Dev Biol. 2016 Oct;58:86-95. doi: 10.1016/j.semcdb.2016.06.007. Epub 2016 Jun 8. Semin Cell Dev Biol. 2016. PMID: 27288568 Free PMC article. Review.
-
Extracellular signal-regulated kinase 2 (ERK-2) mediated phosphorylation regulates nucleo-cytoplasmic shuttling and cell growth control of Ras-associated tumor suppressor protein, RASSF2.Exp Cell Res. 2009 Oct 1;315(16):2775-90. doi: 10.1016/j.yexcr.2009.06.013. Epub 2009 Jun 23. Exp Cell Res. 2009. PMID: 19555684
-
The Ras effector RASSF2 is a novel tumor-suppressor gene in human colorectal cancer.Gastroenterology. 2005 Jul;129(1):156-69. doi: 10.1053/j.gastro.2005.03.051. Gastroenterology. 2005. PMID: 16012945
-
[Methylation and protein expression of RASSF2 in prostate cancer].Zhonghua Nan Ke Xue. 2013 Feb;19(2):107-10. Zhonghua Nan Ke Xue. 2013. PMID: 23441448 Chinese.
-
The RASSF1A tumor suppressor.J Cell Sci. 2007 Sep 15;120(Pt 18):3163-72. doi: 10.1242/jcs.010389. J Cell Sci. 2007. PMID: 17878233 Review.
Cited by
-
Genes related to suppression of malignant phenotype induced by Maitake D-Fraction in breast cancer cells.J Med Food. 2013 Jul;16(7):602-17. doi: 10.1089/jmf.2012.0222. J Med Food. 2013. PMID: 23875900 Free PMC article.
-
Ras signaling through RASSF proteins.Semin Cell Dev Biol. 2016 Oct;58:86-95. doi: 10.1016/j.semcdb.2016.06.007. Epub 2016 Jun 8. Semin Cell Dev Biol. 2016. PMID: 27288568 Free PMC article. Review.
-
Computer-Aided Drug Design Boosts RAS Inhibitor Discovery.Molecules. 2022 Sep 5;27(17):5710. doi: 10.3390/molecules27175710. Molecules. 2022. PMID: 36080477 Free PMC article. Review.
-
Tumor suppressor C-RASSF proteins.Cell Mol Life Sci. 2018 May;75(10):1773-1787. doi: 10.1007/s00018-018-2756-5. Epub 2018 Jan 20. Cell Mol Life Sci. 2018. PMID: 29353317 Free PMC article. Review.
-
Proteomics Analysis Reveals Novel RASSF2 Interaction Partners.Cancers (Basel). 2016 Mar 16;8(3):37. doi: 10.3390/cancers8030037. Cancers (Basel). 2016. PMID: 26999212 Free PMC article.
References
-
- Akino, K., M. Toyota, H. Suzuki, H. Mita, Y. Sasaki, M. Ohe-Toyota, J. P. Issa, Y. Hinoda, K. Imai, and T. Tokino. 2005. The Ras effector RASSF2 is a novel tumor-suppressor gene in human colorectal cancer. Gastroenterology 129:156-169. - PubMed
-
- Boehrer, S., K. U. Chow, E. Puccetti, M. Ruthardt, S. Godzisard, A. Krapohl, B. Schneider, D. Hoelzer, P. S. Mitrou, V. M. Rangnekar, and E. Weidmann. 2001. Deregulated expression of prostate apoptosis response gene-4 in less differentiated lymphocytes and inverse expressional patterns of par-4 and bcl-2 in acute lymphocytic leukemia. Hematol. J. 2:103-107. - PubMed
-
- Boehrer, S., D. Nowak, E. Puccetti, M. Ruthardt, N. Sattler, B. Trepohl, B. Schneider, D. Hoelzer, P. S. Mitrou, and K. U. Chow. 2006. Prostate-apoptosis-response-gene-4 increases sensitivity to TRAIL-induced apoptosis. Leuk. Res. 30:597-605. - PubMed
-
- Calvisi, D. F., S. Ladu, A. Gorden, M. Farina, E. A. Conner, J. S. Lee, V. M. Factor, and S. S. Thorgeirsson. 2006. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology 130:1117-1128. - PubMed
-
- Chakraborty, M., S. G. Qiu, K. M. Vasudevan, and V. M. Rangnekar. 2001. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 61:7255-7263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
