Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection
- PMID: 20368393
- PMCID: PMC2876358
- DOI: 10.1128/AAC.01755-09
Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection
Abstract
We conducted a double-blind, randomized controlled trial to compare a long-acting neuraminidase inhibitor, laninamivir octanoate, with oseltamivir. Eligible patients were children 9 years of age and under who had febrile influenza symptoms of no more than 36-h duration. Patients were randomized to 1 of 3 treatment groups: a group given 40 mg laninamivir (40-mg group), a group given 20 mg laninamivir (20-mg group), and an oseltamivir group. Laninamivir octanoate was administered as a single inhalation. Oseltamivir (2 mg/kg of body weight) was administered orally twice daily for 5 days. The primary end point was the time to alleviation of influenza illness. The primary analysis included 184 patients (61, 61, and 62 in the 40-mg group, 20-mg group, and oseltamivir group, respectively). Laninamivir octanoate markedly reduced the median time to illness alleviation in comparison with oseltamivir in patients infected with oseltamivir-resistant influenza A (H1N1) virus, and the reductions were 60.9 h for the 40-mg group and 66.2 h for the 20-mg group. On the other hand, there were no significant differences in the times to alleviation of illness between the laninamivir groups and oseltamivir group for patients with influenza A (H3N2) or B virus infection. Laninamivir octanoate was well tolerated. The most common adverse events were gastrointestinal events. Laninamivir octanoate was an effective and well-tolerated treatment for children with oseltamivir-resistant influenza A (H1N1) virus infection. Further study will be needed to confirm clinical efficacy against influenza A (H3N2) or B virus infection. Its ease of administration is noteworthy, because a single inhalation is required during the course of illness.
Figures
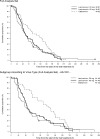
References
-
- Behrens, B., and G. Kärber. 1935. Wie sind Rehenversuche für biologishe Auswertungen am zweckmäsigsten anzuordnen? Naunyn Schmiedebergs Arch. Pharmakol. Exp. Pathol. 177:377-388.
-
- Dominguez-Cherit, G., S. E. Lapinsky, A. E. Macias, et al. 2009. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA 302:1880-1887. - PubMed
-
- Guo, L., R. J. Garten, A. S. Foust, et al. 2009. Rapid identification of oseltamivir-resistant influenza A (H1N1) viruses with H274Y mutation by RT-PCR/restriction fragment length polymorphism assay. Antiviral Res. 82:29-33. - PubMed
-
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1996. ICH harmonized tripartite guideline: guideline for good clinical practice. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland. http://www.ich.org/cache/compo/276-254-1.html.
-
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1998. ICH harmonized tripartite guideline: statistical principles for clinical trials. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland. http://www.ich.org/cache/compo/276-254-1.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

