Pharmacokinetics of sulfobutylether-beta-cyclodextrin and voriconazole in patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration
- PMID: 20368400
- PMCID: PMC2876385
- DOI: 10.1128/AAC.01540-09
Pharmacokinetics of sulfobutylether-beta-cyclodextrin and voriconazole in patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration
Abstract
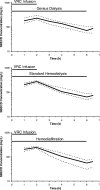
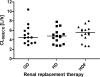
Sulfobutylether-beta-cyclodextrin (SBECD), a large cyclic oligosaccharide that is used to solubilize voriconazole (VRC) for intravenous administration, is eliminated mainly by renal excretion. The pharmacokinetics of SBECD and voriconazole in patients undergoing extracorporeal renal replacement therapies are not well defined. We performed a three-period randomized crossover study of 15 patients with end-stage renal failure during 6-hour treatment with Genius dialysis, standard hemodialysis, or hemodiafiltration using a high-flux polysulfone membrane. At the start of renal replacement therapy, the patients received a single 2-h infusion of voriconazole (4 mg per kg of body weight) solubilized with SBECD. SBECD, voriconazole, and voriconazole-N-oxide concentrations were quantified in plasma and dialysate samples by high-performance liquid chromatography (HPLC) and by HPLC coupled to tandem mass spectrometry (LC-MS-MS) and analyzed by noncompartmental methods. Nonparametric repeated-measures analysis of variance (ANOVA) was used to analyze differences between treatment phases. SBECD and voriconazole recoveries in dialysate samples were 67% and 10% of the administered doses. SBECD concentrations declined with a half-life ranging from 2.6 +/- 0.6 h (Genius dialysis) to 2.4 +/- 0.9 h (hemodialysis) and 2.0 +/- 0.6 h (hemodiafiltration) (P < 0.01 for Genius dialysis versus hemodiafiltration). Prediction of steady-state conditions indicated that even with daily hemodialysis, SBECD will still exceed SBECD exposure of patients with normal renal function by a factor of 6.2. SBECD was effectively eliminated during 6 h of renal replacement therapy by all methods, using high-flux polysulfone membranes, whereas elimination of voriconazole was quantitatively insignificant. The SBECD half-life during renal replacement therapy was nearly normalized, but the average SBECD exposure during repeated administration is expected to be still increased.
Figures

References
-
- Abel, S., R. Allan, K. Gandelman, K. Tomaszewski, D. J. Webb, and N. D. Wood. 2008. Pharmacokinetics, safety and tolerance of voriconazole in renally impaired subjects: two prospective, multicentre, open-label, parallel-group volunteer studies. Clin. Drug Investig. 28:409-420. - PubMed
-
- Bayliss, M. A. J., R. Gage, A. M. Edgington, and R. F. Venn. 2000. Fluorescence determination of sulphobutylether-beta-cyclodextrin sodium in human plasma and urine by size-exclusion chromatography with inclusion complex formation. Chromatographia 52:S83-S86. - PubMed
-
- Fliser, D., and J. T. Kielstein. 2006. Treatment of renal failure in the intensive care unit with extended dialysis. Nat. Clin. Pract. Nephrol. 2:32-39. - PubMed
-
- Gage, R., R. F. Venn, M. A. Bayliss, A. M. Edgington, S. J. Roffey, and B. Sorrell. 2000. Fluorescence determination of sulphobutylether-beta-cyclodextrin in human plasma by size exclusion chromatography with inclusion complex formation. J. Pharm. Biomed. Anal. 22:773-780. - PubMed
-
- Ghannoum, M. A., and D. M. Kuhn. 2002. Voriconazole-better chances for patients with invasive mycoses. Eur. J. Med. Res. 7:242-256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

