Population pharmacokinetics and pharmacodynamic considerations of amodiaquine and desethylamodiaquine in Kenyan adults with uncomplicated malaria receiving artesunate-amodiaquine combination therapy
- PMID: 20368402
- PMCID: PMC2876388
- DOI: 10.1128/AAC.01496-09
Population pharmacokinetics and pharmacodynamic considerations of amodiaquine and desethylamodiaquine in Kenyan adults with uncomplicated malaria receiving artesunate-amodiaquine combination therapy
Abstract
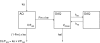
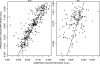
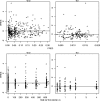
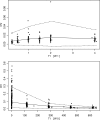
Amodiaquine (AQ) is an antimalarial drug that was frequently combined with artesunate (AS) for the treatment of uncomplicated malaria due to Plasmodium falciparum and is now available as a fixed-dose combination. Despite its widespread use, the simultaneous pharmacokinetics in patients of AQ and its active metabolite, desethylamodiaquine (DAQ), were not characterized to date. The pharmacokinetics of AQ and DAQ in 54 adult patients receiving the AS/AQ combination were therefore investigated by the use of a population approach. AQ followed a 1-compartment model with first-order absorption and elimination, as well as a first-order and irreversible transformation into DAQ, which in turn followed a 2-compartment model with first-order elimination from its central compartment. The mean AQ apparent clearance and distribution volume were 3,410 liters/h and 39,200 liters, respectively. The mean terminal elimination half-life of DAQ was 211 h. Body weight was found to explain the interindividual variability of the apparent volume of distribution of AQ and the elimination rate constant of DAQ. A new dosage form consisting of a fixed-dose combination of AS and AQ was found to have no effect on the pharmacokinetic parameters of AQ and DAQ. All patients achieved parasite clearance within 4 days following the initiation of the treatment, which prevented investigation of the possible relationship between DAQ exposure and treatment outcome. This study provided the first simultaneous pharmacokinetic model for AQ and DAQ.
Figures

References
-
- Adjei, G. O., K. Kristensen, B. Q. Goka, L. C. Hoegberg, M. Alifrangis, O. P. Rodrigues, and J. A. Kurtzhals. 2008. Effect of concomitant artesunate administration and cytochrome P4502C8 polymorphisms on the pharmacokinetics of amodiaquine in Ghanaian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52:4400-4406. - PMC - PubMed
-
- Adjei, G. O., J. A. Kurtzhals, O. P. Rodrigues, M. Alifrangis, L. C. Hoegberg, E. D. Kitcher, E. V. Badoe, R. Lamptey, and B. Q. Goka. 2008. Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up. Malar. J. 7:127. - PMC - PubMed
-
- Ashley, E. A., K. Stepniewska, N. Lindegardh, R. McGready, R. Hutagalung, R. Hae, P. Singhasivanon, N. J. White, and F. Nosten. 2006. Population pharmacokinetic assessment of a new regimen of mefloquine used in combination treatment of uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 50:2281-2285. - PMC - PubMed
-
- Aubouy, A., M. Bakary, A. Keundjian, B. Mbomat, J. R. Makita, F. Migot-Nabias, M. Cot, J. Le Bras, and P. Deloron. 2003. Combination of drug level measurement and parasite genotyping data for improved assessment of amodiaquine and sulfadoxine-pyrimethamine efficacies in treating Plasmodium falciparum malaria in Gabonese children. Antimicrob. Agents Chemother. 47:231-237. - PMC - PubMed
-
- Beal, S. L., and L. B. Sheiner. 1991. NONMEM user's guide. NONMEM Project Group, University of California at San Francisco, San Francisco, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

