IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth
- PMID: 20368414
- PMCID: PMC2867736
- DOI: 10.1073/pnas.0907696107
IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth
Abstract
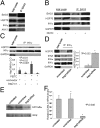
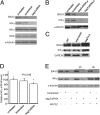
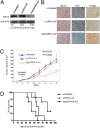
BAG3, a member of the BAG family of heat shock protein (HSP) 70 cochaperones, is expressed in response to stressful stimuli in a number of normal cell types and constitutively in a variety of tumors, including pancreas carcinomas, lymphocytic and myeloblastic leukemias, and thyroid carcinomas. Down-regulation of BAG3 results in cell death, but the underlying molecular mechanisms are still elusive. Here, we investigated the molecular mechanism of BAG3-dependent survival in human osteosarcoma (SAOS-2) and melanoma (M14) cells. We show that bag3 overexpression in tumors promotes survival through the NF-kappaB pathway. Indeed, we demonstrate that BAG3 alters the interaction between HSP70 and IKKgamma, increasing availability of IKKgamma and protecting it from proteasome-dependent degradation; this, in turn, results in increased NF-kappaB activity and survival. These results identify bag3 as a potential target for anticancer therapies in those tumors in which this gene is constitutively expressed. As a proof of principle, we show that treatment of a mouse xenograft tumor model with bag3siRNA-adenovirus that down-regulates bag3 results in reduced tumor growth and increased animal survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. - PubMed
-
- Briknarová K, et al. Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat Struct Biol. 2001;8:349–352. - PubMed
-
- Moribe Y, Niimi T, Yamashita O, Yaginuma T, Samui A. Samui, a novel cold-inducible gene, encoding a protein with a BAG domain similar to silencer of death domains (SODD/BAG-4), isolated from Bombyx diapause eggs. Eur J Biochem. 2001;268:3432–3442. - PubMed
-
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:237–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

