Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis
- PMID: 20368438
- PMCID: PMC2867714
- DOI: 10.1073/pnas.0909707107
Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis
Abstract
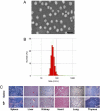
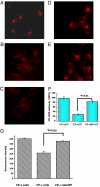
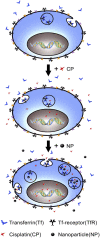
Cisplatin is a chemotherapeutic drug commonly used in clinics. However, acquired resistance confines its application in chemotherapeutics. To overcome the acquired resistance to cisplatin, it is reasoned, based on our previous findings of mediation of cellular responses by [Gd@C(82)(OH)(22)](n) nanoparticles, that [Gd@C(82)(OH)(22)](n) may reverse tumor resistance to cisplatin by reactivating the impaired endocytosis of cisplatin-resistant human prostate cancer (CP-r) cells. Here we report that exposure of the CP-r PC-3-luc cells to cisplatin in the presence of nontoxic [Gd@C(82)(OH)(22)](n) not only decreased the number of surviving CP-r cells but also inhibited growth of the CP-r tumors in athymic nude mice as measured by both optical and MRI. Labeling the CP-r PC-3 cells with transferrin, an endocytotic marker, demonstrated that pretreatment of the CP-r PC-3-luc cells with [Gd@C(82)(OH)(22)](n) enhanced intracellular accumulation of cisplatin and formation of cisplatin-DNA adducts by restoring the defective endocytosis of the CP-r cancer cells. The results suggest that [Gd@C(82)(OH)(22)](n) nanoparticles overcome tumor resistance to cisplatin by increasing its intracellular accumulation through the mechanism of restoring defective endocytosis. The technology can be extended to other challenges related to multidrug resistance often found in cancer treatments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gottlieb JA, Drewinko B. Review of the current clinical status of platinum coordination complexes in cancer chemotherapy. Cancer Chemother Rep. 1975;59:621–628. - PubMed
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. - PubMed
-
- Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. - PubMed
-
- Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. - PubMed
-
- Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

