Development of a multimarker assay for early detection of ovarian cancer
- PMID: 20368574
- PMCID: PMC2860434
- DOI: 10.1200/JCO.2008.19.2484
Development of a multimarker assay for early detection of ovarian cancer
Abstract
Purpose: Early detection of ovarian cancer has great promise to improve clinical outcome.
Patients and methods: Ninety-six serum biomarkers were analyzed in sera from healthy women and from patients with ovarian cancer, benign pelvic tumors, and breast, colorectal, and lung cancers, using multiplex xMAP bead-based immunoassays. A Metropolis algorithm with Monte Carlo simulation (MMC) was used for analysis of the data.
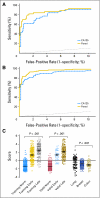
Results: A training set, including sera from 139 patients with early-stage ovarian cancer, 149 patients with late-stage ovarian cancer, and 1,102 healthy women, was analyzed with MMC algorithm and cross validation to identify an optimal biomarker panel discriminating early-stage cancer from healthy controls. The four-biomarker panel providing the highest diagnostic power of 86% sensitivity (SN) for early-stage and 93% SN for late-stage ovarian cancer at 98% specificity (SP) was comprised of CA-125, HE4, CEA, and VCAM-1. This model was applied to an independent blinded validation set consisting of sera from 44 patients with early-stage ovarian cancer, 124 patients with late-stage ovarian cancer, and 929 healthy women, providing unbiased estimates of 86% SN for stage I and II and 95% SN for stage III and IV disease at 98% SP. This panel was selective for ovarian cancer showing SN of 33% for benign pelvic disease, SN of 6% for breast cancer, SN of 0% for colorectal cancer, and SN of 36% for lung cancer.
Conclusion: A panel of CA-125, HE4, CEA, and VCAM-1, after additional validation, could serve as an initial stage in a screening strategy for epithelial ovarian cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
A step forward for two-step screening for ovarian cancer.J Clin Oncol. 2010 May 1;28(13):2128-30. doi: 10.1200/JCO.2009.26.6346. Epub 2010 Apr 5. J Clin Oncol. 2010. PMID: 20368556 No abstract available.
Similar articles
-
A step forward for two-step screening for ovarian cancer.J Clin Oncol. 2010 May 1;28(13):2128-30. doi: 10.1200/JCO.2009.26.6346. Epub 2010 Apr 5. J Clin Oncol. 2010. PMID: 20368556 No abstract available.
-
Human epididymis protein 4 antigen-autoantibody complexes complement cancer antigen 125 for detecting early-stage ovarian cancer.Cancer. 2020 Feb 15;126(4):725-736. doi: 10.1002/cncr.32582. Epub 2019 Nov 12. Cancer. 2020. PMID: 31714597 Free PMC article.
-
Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass.Gynecol Oncol. 2010 Jun;117(3):440-5. doi: 10.1016/j.ygyno.2010.02.005. Epub 2010 Mar 24. Gynecol Oncol. 2010. PMID: 20334903 Free PMC article.
-
HE4 as a biomarker for ovarian and endometrial cancer management.Expert Rev Mol Diagn. 2009 Sep;9(6):555-66. doi: 10.1586/erm.09.39. Expert Rev Mol Diagn. 2009. PMID: 19732003 Free PMC article. Review.
-
The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125?Am J Obstet Gynecol. 2008 Sep;199(3):215-23. doi: 10.1016/j.ajog.2008.04.009. Epub 2008 May 12. Am J Obstet Gynecol. 2008. PMID: 18468571 Review.
Cited by
-
Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response.Diagn Pathol. 2013 Jan 23;8:11. doi: 10.1186/1746-1596-8-11. Diagn Pathol. 2013. PMID: 23343214 Free PMC article.
-
DR6 as a diagnostic and predictive biomarker in adult sarcoma.PLoS One. 2012;7(5):e36525. doi: 10.1371/journal.pone.0036525. Epub 2012 May 2. PLoS One. 2012. PMID: 22567163 Free PMC article.
-
An Assessment of Novel Biomarkers in Bone Metastatic Disease Using Multiplex Measurement and Multivariate Analysis.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818807466. doi: 10.1177/1533033818807466. Technol Cancer Res Treat. 2018. PMID: 30343636 Free PMC article.
-
Novel protein and immune response markers of human serous tubal intraepithelial carcinoma of the ovary.Cancer Biomark. 2019;26(4):471-479. doi: 10.3233/CBM-190528. Cancer Biomark. 2019. PMID: 31658047 Free PMC article.
-
Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells.Int J Mol Sci. 2014 Feb 27;15(3):3560-79. doi: 10.3390/ijms15033560. Int J Mol Sci. 2014. PMID: 24583847 Free PMC article.
References
-
- Boente MP, Schilder R, Ozols RF. Gynecological cancers. Cancer Chemother Biol Response Modif. 1999;18:418–434. - PubMed
-
- Baker TR, Piver MS. Etiology, biology, and epidemiology of ovarian cancer. Semin Surg Oncol. 1994;10:242–248. - PubMed
-
- Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. - PubMed
-
- Jacobs I, Bast RC., Jr The CA 125 tumour-associated antigen: A review of the literature. Hum Reprod. 1989;4:1–12. - PubMed
-
- van Haaften-Day C, Shen Y, Xu F, et al. OVX1, macrophage-colony stimulating factor, and CA-125-II as tumor markers for epithelial ovarian carcinoma: A critical appraisal. Cancer. 2001;92:2837–2844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

