Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates
- PMID: 20368967
- PMCID: PMC2848555
- DOI: 10.1371/journal.ppat.1000832
Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates
Abstract
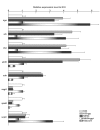
Streptococcal toxic shock syndrome (STSS) is a severe invasive infection characterized by the sudden onset of shock and multiorgan failure; it has a high mortality rate. Although a number of studies have attempted to determine the crucial factors behind the onset of STSS, the responsible genes in group A Streptococcus have not been clarified. We previously reported that mutations of csrS/csrR genes, a two-component negative regulator system for multiple virulence genes of Streptococcus pyogenes, are found among the isolates from STSS patients. In the present study, mutations of another negative regulator, rgg, were also found in clinical isolates of STSS patients. The rgg mutants from STSS clinical isolates enhanced lethality and impaired various organs in the mouse models, similar to the csrS mutants, and precluded their being killed by human neutrophils, mainly due to an overproduction of SLO. When we assessed the mutation frequency of csrS, csrR, and rgg genes among S. pyogenes isolates from STSS (164 isolates) and non-invasive infections (59 isolates), 57.3% of the STSS isolates had mutations of one or more genes among three genes, while isolates from patients with non-invasive disease had significantly fewer mutations in these genes (1.7%). The results of the present study suggest that mutations in the negative regulators csrS/csrR and rgg of S. pyogenes are crucial factors in the pathogenesis of STSS, as they lead to the overproduction of multiple virulence factors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


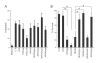
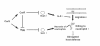
References
-
- Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. - PubMed
-
- Ato M, Ikebe T, Kawabata H, Takemori T, Watanabe H. Incompetence of neutrophils to invasive group A streptococcus is attributed to induction of plural virulence factors by dysfunction of a regulator. PLoS ONE. 2008;3:e3455. doi: 10.1371/journal.pone.0003455. - DOI - PMC - PubMed
-
- Walker M J, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. - PubMed
-
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. - DOI - PMC - PubMed
-
- Miyoshi-Akiyama T, Ikebe T, Watanabe H, Uchiyama T, Kirikae T, et al. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J Infect Dis. 2006;193:1677–1684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

