The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti
- PMID: 20368968
- PMCID: PMC2848556
- DOI: 10.1371/journal.ppat.1000833
The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti
Abstract
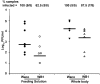
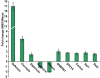
Genetic strategies that reduce or block pathogen transmission by mosquitoes have been proposed as a means of augmenting current control measures to reduce the growing burden of vector-borne diseases. The endosymbiotic bacterium Wolbachia has long been promoted as a potential vehicle for introducing disease-resistance genes into mosquitoes, thereby making them refractory to the human pathogens they transmit. Given the large overlap in tissue distribution and intracellular localization between Wolbachia and dengue virus in mosquitoes, we conducted experiments to characterize their interactions. Our results show that Wolbachia inhibits viral replication and dissemination in the main dengue vector, Aedes aegypti. Moreover, the virus transmission potential of Wolbachia-infected Ae. aegypti was significantly diminished when compared to wild-type mosquitoes that did not harbor Wolbachia. At 14 days post-infection, Wolbachia completely blocked dengue transmission in at least 37.5% of Ae. aegypti mosquitoes. We also observed that this Wolbachia-mediated viral interference was associated with an elevated basal immunity and increased longevity in the mosquitoes. These results underscore the potential usefulness of Wolbachia-based control strategies for population replacement.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


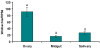

References
-
- Gubler DJ, Kuno G. Dengue and Dengue Hemorrhagic Fever. New York Cab International; 1997.
-
- Olson KE, Higgs S, Gaines PJ, Powers AM, Davis BS, et al. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. - PubMed
-
- Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. - PubMed
-
- Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat Rev Genet. 2006;7:427–435. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

