Characterization of aziridinylbenzoquinone DNA cross-links by liquid chromatography-infrared multiphoton dissociation-mass spectrometry
- PMID: 20369834
- PMCID: PMC2891125
- DOI: 10.1021/tx1000738
Characterization of aziridinylbenzoquinone DNA cross-links by liquid chromatography-infrared multiphoton dissociation-mass spectrometry
Abstract
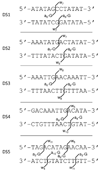
DNA cross-linking was evaluated by liquid chromatography-tandem mass spectrometry to determine the relative cross-linking abilities of two aziridinylbenzoquinones. Reactivities of RH1 (2,5-diaziridinyl-3-[hydroxymethyl]-6-methyl-1,4-benzoquinone), a clinically studied antitumor cross-linking agent, and an analogue containing a phenyl group (2,5-diaziridinyl-3-[hydroxymethyl]-6-phenyl-1,4-benzoquinone, PhRH1) rather than a methyl group were compared. The bulky phenyl substituent was added to determine the impact of steric hindrance on the formation of cross-links within a double helical structure. Cross-links formed by RH1 and PhRH1 were observed at 5'-dGNC sites as well as 5'-dGAAC/dGTTC sites. RH1 was more effective at forming cross-links than PhRH1 for a variety of duplexes. Infrared multiphoton dissociation (IRMPD) and collision-induced dissociation results confirmed the presence and the location of the cross-links within the duplexes, and IRMPD was used to identify the dissociation pathways of the cross-linked duplexes.
Figures








Similar articles
-
DNA cross-linking and sequence selectivity of aziridinylbenzoquinones: a unique reaction at 5'-GC-3' sequences with 2,5-diaziridinyl-1,4-benzoquinone upon reduction.Biochemistry. 1991 Dec 17;30(50):11719-24. doi: 10.1021/bi00114a016. Biochemistry. 1991. PMID: 1751490
-
Preclinical evaluation of the pharmacodynamic properties of 2,5-diaziridinyl-3-hydroxymethyl-6-methyl-1,4-benzoquinone.Clin Cancer Res. 2005 Apr 1;11(7):2695-701. doi: 10.1158/1078-0432.CCR-04-1751. Clin Cancer Res. 2005. PMID: 15814651
-
Two structurally related diaziridinylbenzoquinones preferentially cross-link DNA at different sites upon reduction with DT-diaphorase.Biochemistry. 1993 Apr 6;32(13):3306-12. doi: 10.1021/bi00064a013. Biochemistry. 1993. PMID: 8461296
-
DNA interstrand cross-linking agents and their chemotherapeutic potential.Curr Med Chem. 2012;19(3):364-85. doi: 10.2174/092986712803414295. Curr Med Chem. 2012. PMID: 22335513 Review.
-
Preparation of interstrand cross-linked DNA oligonucleotide duplexes.Front Biosci. 2004 Jan 1;9:421-37. doi: 10.2741/1246. Front Biosci. 2004. PMID: 14766379 Review.
Cited by
-
Comparison of MS/MS methods for characterization of DNA/cisplatin adducts.J Am Soc Mass Spectrom. 2013 Feb;24(2):265-73. doi: 10.1007/s13361-012-0532-6. Epub 2012 Dec 20. J Am Soc Mass Spectrom. 2013. PMID: 23264150 Free PMC article.
-
Tandem mass spectrometry for characterization of covalent adducts of DNA with anticancer therapeutics.Mass Spectrom Rev. 2013 Jul-Aug;32(4):247-66. doi: 10.1002/mas.21363. Epub 2012 Nov 13. Mass Spectrom Rev. 2013. PMID: 23150278 Free PMC article. Review.
-
Dual-Action Therapeutics: DNA Alkylation and Antimicrobial Peptides for Cancer Therapy.Cancers (Basel). 2024 Sep 10;16(18):3123. doi: 10.3390/cancers16183123. Cancers (Basel). 2024. PMID: 39335095 Free PMC article. Review.
-
Hypoxia-active nanoparticles used in tumor theranostic.Int J Nanomedicine. 2019 May 22;14:3705-3722. doi: 10.2147/IJN.S196959. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31190820 Free PMC article. Review.
-
Photodissociation mass spectrometry: new tools for characterization of biological molecules.Chem Soc Rev. 2014 Apr 21;43(8):2757-83. doi: 10.1039/c3cs60444f. Epub 2014 Jan 30. Chem Soc Rev. 2014. PMID: 24481009 Free PMC article. Review.
References
-
- Martinez R, Chacon-Garcia L. The Search of DNA-Intercalators as Antitumoral Drugs: What Worked and What Did Not Work. Curr. Med. Chem. 2005;12:127–151. - PubMed
-
- Ferguson LR, Denny WA. Genotoxicity of Non-Covalent Interactions: DNA Intercalators. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2007;623:14–23. - PubMed
-
- Nelson SM, Ferguson LR, Denny WA. Non-Covalent Ligand/DNA Interactions: Minor Groove Binding Agents. Mutat. Res.-Fundam. Mol. Mech. Mutag. 2007;623:24–40. - PubMed
-
- Li HH, Aubrecht J, Fornace AJ. Toxicogenomics: Overview and Potential Applications for the Study of Non-Covalent DNA Interacting Chemicals. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2007;623:98–108. - PubMed
-
- Auclair C, Dugue B, Meunier B, Paoletti C. Peroxidase-Catalyzed Covalent Binding of the Antitumor Drug N2-Methyl-9-Hydroxyellipticinium to DNA in Vitro. Biochemistry. 1986;25:1240–1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

