Reactive oxygen species in cancer
- PMID: 20370557
- PMCID: PMC3880197
- DOI: 10.3109/10715761003667554
Reactive oxygen species in cancer
Abstract
Elevated rates of reactive oxygen species (ROS) have been detected in almost all cancers, where they promote many aspects of tumour development and progression. However, tumour cells also express increased levels of antioxidant proteins to detoxify from ROS, suggesting that a delicate balance of intracellular ROS levels is required for cancer cell function. Further, the radical generated, the location of its generation, as well as the local concentration is important for the cellular functions of ROS in cancer. A challenge for novel therapeutic strategies will be the fine tuning of intracellular ROS signalling to effectively deprive cells from ROS-induced tumour promoting events, towards tipping the balance to ROS-induced apoptotic signalling. Alternatively, therapeutic antioxidants may prevent early events in tumour development, where ROS are important. However, to effectively target cancer cells specific ROS-sensing signalling pathways that mediate the diverse stress-regulated cellular functions need to be identified. This review discusses the generation of ROS within tumour cells, their detoxification, their cellular effects, as well as the major signalling cascades they utilize, but also provides an outlook on their modulation in therapeutics.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the review.
Figures
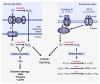


References
-
- Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. - PubMed
-
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–8. - PubMed
-
- Babio BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
