Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses
- PMID: 20370617
- PMCID: PMC3038989
- DOI: 10.1615/critrevimmunol.v30.i1.10
Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses
Abstract
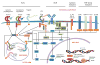
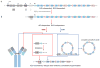
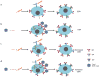
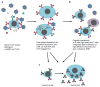
Differentiation of naïve B cells, including immunoglobulin class-switch DNA recombination, is critical for the immune response and depends on the extensive integration of signals from the B-cell receptor (BCR), tumor necrosis factor (TNF) family members, Toll-like receptors (TLRs), and cytokine receptors. TLRs and BCR synergize to induce class-switch DNA recombination in T cell-dependent and T cell-independent antibody responses to microbial pathogens. BCR triggering together with simultaneous endosomal TLR engagement leads to enhanced B-cell differentiation and antibody responses. Te requirement of both BCR and TLR engagement would ensure appropriate antigen-specific activation in an infection. Co-stimulation of TLRs and BCR likely plays a significant role in anti-microbial antibody responses to contain pathogen loads until the T cell-dependent antibody responses peak. Furthermore, the temporal sequence of different signals is also critical for optimal B cell responses, as exemplified by the activation of B cells by initial TLR engagement, leading to the up-regulation of co-stimulatory CD80 and MCH-II receptors, which result in more efficient interactions with T cells, thereby enhancing the germinal center reaction and antibody affinity maturation. Overall, BCR and TLR stimulation and the integration with signals from the pathogen or immune cells and their products determine the ensuing B-cell antibody response.
Figures

Similar articles
-
B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: modulation by BCR and CD40, and relevance to T-independent antibody responses.Autoimmunity. 2015 Feb;48(1):1-12. doi: 10.3109/08916934.2014.993027. Autoimmunity. 2015. PMID: 25536171 Free PMC article.
-
How the Signaling Crosstalk of B Cell Receptor (BCR) and Co-Receptors Regulates Antibody Class Switch Recombination: A New Perspective of Checkpoints of BCR Signaling.Front Immunol. 2021 Mar 25;12:663443. doi: 10.3389/fimmu.2021.663443. eCollection 2021. Front Immunol. 2021. PMID: 33841447 Free PMC article. Review.
-
B cell TLRs and induction of immunoglobulin class-switch DNA recombination.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2594-615. doi: 10.2741/4073. Front Biosci (Landmark Ed). 2012. PMID: 22652800 Free PMC article. Review.
-
BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway.Nat Commun. 2012 Apr 3;3:767. doi: 10.1038/ncomms1769. Nat Commun. 2012. PMID: 22473011 Free PMC article.
-
Control of Toll-like receptor-mediated T cell-independent type 1 antibody responses by the inducible nuclear protein IκB-ζ.J Biol Chem. 2014 Nov 7;289(45):30925-36. doi: 10.1074/jbc.M114.553230. Epub 2014 Aug 14. J Biol Chem. 2014. PMID: 25124037 Free PMC article.
Cited by
-
Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection.J Virol. 2012 Nov;86(21):11533-40. doi: 10.1128/JVI.00615-12. Epub 2012 Aug 15. J Virol. 2012. PMID: 22896601 Free PMC article.
-
Immunoglobulin G structure and rheumatoid factor epitopes.PLoS One. 2019 Jun 14;14(6):e0217624. doi: 10.1371/journal.pone.0217624. eCollection 2019. PLoS One. 2019. PMID: 31199818 Free PMC article.
-
IgG subclasses and allotypes: from structure to effector functions.Front Immunol. 2014 Oct 20;5:520. doi: 10.3389/fimmu.2014.00520. eCollection 2014. Front Immunol. 2014. PMID: 25368619 Free PMC article. Review.
-
Key Considerations for the Development of Safe and Effective SARS-CoV-2 Subunit Vaccine: A Peptide-Based Vaccine Alternative.Adv Sci (Weinh). 2021 Aug;8(16):e2100985. doi: 10.1002/advs.202100985. Epub 2021 Jun 27. Adv Sci (Weinh). 2021. PMID: 34176237 Free PMC article. Review.
-
Intravital quantification reveals dynamic calcium concentration changes across B cell differentiation stages.Elife. 2021 Mar 22;10:e56020. doi: 10.7554/eLife.56020. Elife. 2021. PMID: 33749591 Free PMC article.
References
-
- Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25:640–644. - PubMed
-
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. - PubMed
-
- Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009;10:333–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

