Treatment of hepatitis C virus infection with interferon and small molecule direct antivirals: viral kinetics and modeling
- PMID: 20370626
- PMCID: PMC2882097
- DOI: 10.1615/critrevimmunol.v30.i2.30
Treatment of hepatitis C virus infection with interferon and small molecule direct antivirals: viral kinetics and modeling
Abstract
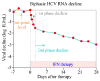
Hepatitis C virus (HCV) infection remains a threat to global public health. Treatment with pegylated interferon (IFN) plus ribavirin leads to a sustained virologic response in about 50% of patients. New therapies using direct antiviral agents have the potential to cure patients unresponsive to IFN-based therapies. Mathematical modeling has played an important role in studying HCV kinetics. Using models, one can evaluate the effectiveness of new treatment agents, estimate important parameters that govern virus-host interactions, explore possible mechanisms of drug action against HCV, investigate the development of drug resistance, and study quasispecies dynamics during therapy. Here we review our current knowledge of HCV kinetics under IFN-based therapy and newly developed antiviral agents specifically targeted to attack HCV, and show how mathematical models have helped to improve our understanding of HCV infection and treatment.
Figures
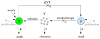




References
-
- World Health Organization. Hepatitis C. Fact sheet No. 164. Revised. Oct, 2000. http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
-
- Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–2. - PubMed
-
- Sharma P, Lok A. Viral hepatitis and liver transplantation. Semin Liver Dis. 2006;26:285–97. - PubMed
-
- Foster GR. Past, present, and future hepatitis C treatments. Semin Liver Dis. 2004;24 2:97–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

