A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides
- PMID: 20370717
- PMCID: PMC2944655
- DOI: 10.1042/BJ20100129
A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides
Abstract
PtdIns5P was discovered in 1997 [Rameh, Tolias, Duckworth and Cantley (1997) Nature 390, 192-196], but still very little is known about its regulation and function. Hitherto, studies of PtdIns5P regulation have been hindered by the inability to measure cellular PtdIns5P using conventional HPLC, owing to poor separation from PtdIns4P. In the present paper we describe a new HPLC method for resolving PtdIns5P from PtdIns4P, which makes possible accurate measurements of basal and inducible levels of cellular PtdIns5P in the context of other phosphoinositides. Using this new method, we found that PtdIns5P is constitutively present in all cells examined (epithelial cells, fibroblasts and myoblasts, among others) at levels typically 1-2% of PtdIns4P levels. In the beta-pancreatic cell line BTC6, which is specialized in insulin secretion, PtdIns5P levels were higher than in most cells (2.5-4% of PtdIns4P). Using subcellular fractionation, we found that the majority of the basal PtdIns5P is present in the plasma membrane, but it is also enriched in intracellular membrane compartments, especially in SER (smooth endoplasmic reticulum) and/or Golgi, where high levels of PtdIns3P were also detected. Unlike PtdIns3P, PtdIns5P was also found in fractions containing very-low-density vesicles. Knockdown of PIP4K (PtdIns5P 4-kinase) leads to accumulation of PtdIns5P in light fractions and fractions enriched in SER/Golgi, whereas treatment with Brefeldin A results in a subtle, but reproducible, change in PtdIns5P distribution. These results indicate that basal PtdIns5P and the PtdIns5P pathway for PtdIns(4,5)P(2) synthesis may play a role in Golgi-mediated vesicle trafficking.
Figures



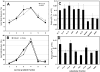

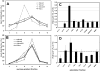

Comment in
-
New methods for capturing the mystery lipid, PtdIns5P.Biochem J. 2010 May 27;428(3):e1-2. doi: 10.1042/BJ20100688. Biochem J. 2010. PMID: 20504279 Free PMC article.
References
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. - PubMed
-
- Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate [see comments] Nature. 1997;390:192–6. - PubMed
-
- Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, Heck AJ, D’Santos CS, Divecha N. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell. 2006;23:685–95. - PubMed
-
- Sbrissa D, Ikonomov OC, Strakova J, Shisheva A. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology. 2004;145:4853–65. - PubMed
-
- Guittard G, Gerard A, Dupuis-Coronas S, Tronchere H, Mortier E, Favre C, Olive D, Zimmermann P, Payrastre B, Nunes JA. Cutting edge: Dok-1 and Dok-2 adaptor molecules are regulated by phosphatidylinositol 5-phosphate production in T cells. J Immunol. 2009;182:3974–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

