Platelet function and Isoprostane biology. Should isoprostanes be the newest member of the orphan-ligand family?
- PMID: 20370921
- PMCID: PMC2854111
- DOI: 10.1186/1423-0127-17-24
Platelet function and Isoprostane biology. Should isoprostanes be the newest member of the orphan-ligand family?
Abstract
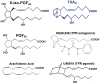
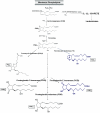
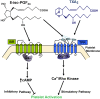
While there have been many reports investigating the biological activity and signaling mechanisms of isoprostanes, their role in biology, particularly in platelets, appears to still be underestimated. Moreover, whether these lipids have their own receptors is still debated, despite multiple reports that discrete receptors for isoprostane do exist on platelets, vascular tissues, amongst others. This paper provides a review of the important literature of isoprostanes and provides reasoning that isoprostanes should be classified as orphan ligands until their receptor(s) is/are identified.
Figures
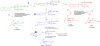
References
-
- Zucker MB, Nachmias VT. Platelet activation. Arteriosclerosis. 1985;5:2–18. - PubMed
-
- Buller HR, Ten Cate T. Coagulation and platelet activation pathways. A review of the key components and the way in which these can be manipulated. Eur Heart J. 1995;16(Suppl L):8–10. - PubMed
-
- Bick RL. Platelet function defects: a clinical review. Semin Thromb Hemost. 1992;18:167–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

