Phospholemman modulates the gating of cardiac L-type calcium channels
- PMID: 20371314
- PMCID: PMC2849072
- DOI: 10.1016/j.bpj.2009.11.032
Phospholemman modulates the gating of cardiac L-type calcium channels
Abstract
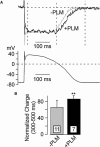
Ca(2+) entry through L-type calcium channels (Ca(V)1.2) is critical in shaping the cardiac action potential and initiating cardiac contraction. Modulation of Ca(V)1.2 channel gating directly affects myocyte excitability and cardiac function. We have found that phospholemman (PLM), a member of the FXYD family and regulator of cardiac ion transport, coimmunoprecipitates with Ca(V)1.2 channels from guinea pig myocytes, which suggests PLM is an endogenous modulator. Cotransfection of PLM in HEK293 cells slowed Ca(V)1.2 current activation at voltages near the threshold for activation, slowed deactivation after long and strong depolarizing steps, enhanced the rate and magnitude of voltage-dependent inactivation (VDI), and slowed recovery from inactivation. However, Ca(2+)-dependent inactivation was not affected. Consistent with slower channel closing, PLM significantly increased Ca(2+) influx via Ca(V)1.2 channels during the repolarization phase of a human cardiac action potential waveform. Our results support PLM as an endogenous regulator of Ca(V)1.2 channel gating. The enhanced VDI induced by PLM may help protect the heart under conditions such as ischemia or tachycardia where the channels are depolarized for prolonged periods of time and could induce Ca(2+) overload. The time and voltage-dependent slowed deactivation could represent a gating shift that helps maintain Ca(2+) influx during the cardiac action potential waveform plateau phase.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Hille B. Sinauer; Sunderland, MA: 2001. Ion Channels of Excitable Membranes.
-
- Splawski I., Timothy K.W., Keating M.T. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

