Stationary gating of GluN1/GluN2B receptors in intact membrane patches
- PMID: 20371315
- PMCID: PMC2849062
- DOI: 10.1016/j.bpj.2009.12.4276
Stationary gating of GluN1/GluN2B receptors in intact membrane patches
Abstract
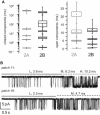
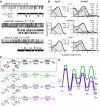
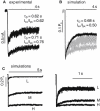
NMDA receptors are heteromeric glutamate-gated channels composed of GluN1 and GluN2 subunits. Receptor isoforms that differ in their GluN2-subunit type (A-D) are expressed differentially throughout the central nervous system and have distinct kinetic properties in recombinant systems. How specific receptor isoforms contribute to the functions generally attributed to NMDA receptors remains unknown, due in part to the incomplete functional characterization of individual receptor types and unclear molecular composition of native receptors. We examined the stationary gating kinetics of individual rat recombinant GluN1/GluN2B receptors in cell-attached patches of transiently transfected HEK293 cells and used kinetic analyses and modeling to describe the full range of this receptor's gating behaviors. We found that, like GluN1/GluN2A receptors, GluN1/GluN2B receptors have three gating modes that are distinguishable by their mean open durations. However, for GluN1/GluN2B receptors, the modes also differed markedly in their mean closed durations and thus generated a broader range of open probabilities. We also found that regardless of gating mode, glutamate dissociation occurred approximately 4-fold more slowly (k(-) = 15 s(-1)) compared to that observed in GluN1/GluN2A receptors. On the basis of these results, we suggest that slow glutamate dissociation and modal gating underlie the long heterogeneous activations of GluN1/GluN2B receptors.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Collingridge G.L., Lester R.A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol. Rev. 1989;41:143–210. - PubMed
-
- McBain C.J., Mayer M.L. N-methyl-d-aspartic acid receptor structure and function. Physiol. Rev. 1994;74:723–760. - PubMed
-
- Sprengel R., Single F.N. Mice with genetically modified NMDA and AMPA receptors. Ann. N. Y. Acad. Sci. 1999;868:494–501. - PubMed
-
- Lester R.A., Clements J.D., Jahr C.E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. - PubMed
-
- Li J.H., Wang Y.H., Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur. J. Neurosci. 1998;10:1704–1715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

