A mathematical analysis of agonist- and KCl-induced Ca(2+) oscillations in mouse airway smooth muscle cells
- PMID: 20371316
- PMCID: PMC2849087
- DOI: 10.1016/j.bpj.2009.12.4273
A mathematical analysis of agonist- and KCl-induced Ca(2+) oscillations in mouse airway smooth muscle cells
Abstract
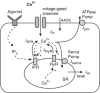
Airway hyperresponsiveness is a major characteristic of asthma and is generally ascribed to excessive airway narrowing associated with the contraction of airway smooth muscle cells (ASMCs). ASMC contraction is initiated by a rise in intracellular calcium concentration ([Ca(2+)](i)), observed as oscillatory Ca(2+) waves that can be induced by either agonist or high extracellular K(+) (KCl). In this work, we present a model of oscillatory Ca(2+) waves based on experimental data that incorporate both the inositol trisphosphate receptor and the ryanodine receptor. We then combined this Ca(2+) model and our modified actin-myosin cross-bridge model to investigate the role and contribution of oscillatory Ca(2+) waves to contractile force generation in mouse ASMCs. The model predicts that: 1), the difference in behavior of agonist- and KCl-induced Ca(2+) waves results principally from the fact that the sarcoplasmic reticulum is depleted during agonist-induced oscillations, but is overfilled during KCl-induced oscillations; 2), regardless of the order in which agonist and KCl are added into the cell, the resulting [Ca(2+)](i) oscillations will always be the short-period, agonist-induced-like oscillations; and 3), both the inositol trisphosphate receptor and the ryanodine receptor densities are higher toward one end of the cell. In addition, our results indicate that oscillatory Ca(2+) waves generate less contraction than whole-cell Ca(2+) oscillations induced by the same agonist concentration. This is due to the spatial inhomogeneity of the receptor distributions.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- de Lanerolle P., Condit J.R., Jr., Adelstein R.S. Myosin phosphorylation, agonist concentration and contraction of tracheal smooth muscle. Nature. 1982;298:871–872. - PubMed
-
- Gerthoffer W.T. Regulation of the contractile element of airway smooth muscle. Am. J. Physiol. 1991;261:15–28. - PubMed
-
- Somlyo A.P., Somlyo A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. - PubMed
-
- Pabelick C.M., Sieck G.C., Prakash Y.S. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J. Appl. Physiol. 2001;91:488–496. - PubMed
-
- Kuo K.H., Dai J., van Breemen C. Relationship between asynchronous Ca2+ waves and force development in intact smooth muscle bundles of the porcine trachea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1345–L1353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

