Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels
- PMID: 20371317
- PMCID: PMC2849097
- DOI: 10.1016/j.bpj.2009.12.4293
Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels
Abstract
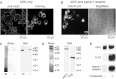
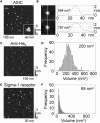
The sigma-1 receptor is a widely expressed protein that interacts with a variety of ion channels, including the acid-sensing ion channel (ASIC) 1a. Here we used atomic force microscopy to determine the architecture of the ASIC1a/sigma-1 receptor complex. When isolated His(8)-tagged ASIC1a was imaged in complex with anti-His(6) antibodies, the angle between pairs of bound antibodies was 135 degrees , consistent with the known trimeric structure of the channel. When ASIC1a was coexpressed with FLAG/His(6)-tagged sigma-1 receptor, ASIC1a became decorated with small particles, and pairs of these particles bound at an angle of 131 degrees . When these complexes were incubated with anti-FLAG antibodies, pairs of antibodies bound at an angle of 134 degrees , confirming that the small particles were sigma-1 receptors. Of interest, we found that the sigma-1 receptor ligand haloperidol caused an approximately 50% reduction in ASIC1a/sigma-receptor binding, suggesting a way in which sigma-1 ligands might modulate channel properties. For the first time, to our knowledge, we have resolved the structure of a complex between the sigma-1 receptor and a target ion channel, and demonstrated that the stoichiometry of the interaction is 1 sigma-1 receptor/1 ASIC1a subunit.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry.J Biol Chem. 2012 Oct 26;287(44):37021-9. doi: 10.1074/jbc.M112.382077. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952230 Free PMC article.
-
A direct interaction between the sigma-1 receptor and the hERG voltage-gated K+ channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF®).J Biol Chem. 2014 Nov 14;289(46):32353-32363. doi: 10.1074/jbc.M114.603506. Epub 2014 Sep 29. J Biol Chem. 2014. PMID: 25266722 Free PMC article.
-
Direct visualization of the trimeric structure of the ASIC1a channel, using AFM imaging.Biochem Biophys Res Commun. 2008 Aug 8;372(4):752-5. doi: 10.1016/j.bbrc.2008.05.100. Epub 2008 Jun 2. Biochem Biophys Res Commun. 2008. PMID: 18514062
-
Acid sensing ion channels--novel therapeutic targets for ischemic brain injury.Front Biosci. 2007 Jan 1;12:1376-86. doi: 10.2741/2154. Front Biosci. 2007. PMID: 17127388 Review.
-
Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure.IUBMB Life. 2008 Sep;60(9):620-8. doi: 10.1002/iub.89. IUBMB Life. 2008. PMID: 18459164 Review.
Cited by
-
The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry.J Biol Chem. 2012 Oct 26;287(44):37021-9. doi: 10.1074/jbc.M112.382077. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952230 Free PMC article.
-
The sigma-1 receptor: a regulator of cancer cell electrical plasticity?Front Physiol. 2013 Jul 16;4:175. doi: 10.3389/fphys.2013.00175. eCollection 2013. Front Physiol. 2013. PMID: 23882221 Free PMC article.
-
Molecular imaging of membrane proteins and microfilaments using atomic force microscopy.Exp Mol Med. 2010 Sep 30;42(9):597-605. doi: 10.3858/emm.2010.42.9.064. Exp Mol Med. 2010. PMID: 20689364 Free PMC article. Review.
-
Sigma-1 Receptor Antagonists Haloperidol and Chlorpromazine Modulate the Effect of Glutoxim on Na+ Transport in Frog Skin.Dokl Biochem Biophys. 2019 May;484(1):63-65. doi: 10.1134/S1607672919010186. Epub 2019 Apr 22. Dokl Biochem Biophys. 2019. PMID: 31012016
-
A direct interaction between the sigma-1 receptor and the hERG voltage-gated K+ channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF®).J Biol Chem. 2014 Nov 14;289(46):32353-32363. doi: 10.1074/jbc.M114.603506. Epub 2014 Sep 29. J Biol Chem. 2014. PMID: 25266722 Free PMC article.
References
-
- Martin W.R., Eades C.G., Gilbert P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. - PubMed
-
- Monnet F.P. σ-1 Receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol. Cell. 2005;97:873–883. - PubMed
-
- Aydar E., Palmer C.P., Jackson M.B. The σ receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. - PubMed
-
- Hayashi T., Su T.P. σ-1 Receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

