Chemo-mechanical coupling in F(1)-ATPase revealed by catalytic site occupancy during catalysis
- PMID: 20371322
- PMCID: PMC2849061
- DOI: 10.1016/j.bpj.2009.11.050
Chemo-mechanical coupling in F(1)-ATPase revealed by catalytic site occupancy during catalysis
Abstract
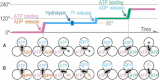
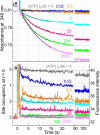
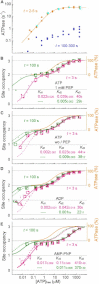
F(1)-ATPase is a rotary molecular motor in which the central gamma subunit rotates inside a cylinder made of alpha(3)beta(3) subunits. To clarify how ATP hydrolysis in three catalytic sites cooperate to drive rotation, we measured the site occupancy, the number of catalytic sites occupied by a nucleotide, while assessing the hydrolysis activity under identical conditions. The results show hitherto unsettled timings of ADP and phosphate releases: starting with ATP binding to a catalytic site at an ATP-waiting gamma angle defined as 0 degrees , phosphate is released at approximately 200 degrees , and ADP is released during quick rotation between 240 degrees and 320 degrees that is initiated by binding of a third ATP. The site occupancy remains two except for a brief moment after the ATP binding, but the third vacant site can bind a medium nucleotide weakly.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Boyer P.D., Kohlbrenner W.E. The present status of the binding-change mechanism and its relation to ATP formation by chloroplasts. In: Selman B.R., Selman-Reimer S., editors. Energy Coupling in Photosynthesis. Elsevier; Amsterdam: 1981. pp. 231–240.
-
- Oosawa F., Hayashi S. The loose coupling mechanism in molecular machines of living cells. Adv. Biophys. 1986;22:151–183. - PubMed
-
- Noji H., Yasuda R., Kinosita K., Jr. Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. - PubMed
-
- Abrahams J.P., Leslie A.G.W., Walker J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
-
- Yasuda R., Noji H., Yoshida M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell. 1998;93:1117–1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

