Matrix metalloproteinases: regulators of the tumor microenvironment
- PMID: 20371345
- PMCID: PMC2862057
- DOI: 10.1016/j.cell.2010.03.015
Matrix metalloproteinases: regulators of the tumor microenvironment
Abstract
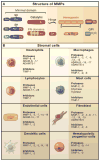
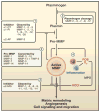
Extracellular proteolysis mediates tissue homeostasis. In cancer, altered proteolysis leads to unregulated tumor growth, tissue remodeling, inflammation, tissue invasion, and metastasis. The matrix metalloproteinases (MMPs) represent the most prominent family of proteinases associated with tumorigenesis. Recent technological developments have markedly advanced our understanding of MMPs as modulators of the tumor microenvironment. In addition to their role in extracellular matrix turnover and cancer cell migration, MMPs regulate signaling pathways that control cell growth, inflammation, or angiogenesis and may even work in a nonproteolytic manner. These aspects of MMP function are reorienting our approaches to cancer therapy.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 Proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

