Resiliency and vulnerability in the HER2-HER3 tumorigenic driver
- PMID: 20371474
- PMCID: PMC3033659
- DOI: 10.1126/scitranslmed.3000389
Resiliency and vulnerability in the HER2-HER3 tumorigenic driver
Abstract
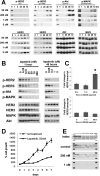
About 25% of breast cancers harbor the amplified oncogene human epidermal growth factor receptor 2 (HER2) and are dependent on HER2 kinase function, identifying HER2 as a vulnerable target for therapy. However, HER2-HER3 signaling is buffered so that it is protected against a nearly two-log inhibition of HER2 catalytic activity; this buffering is driven by the negative regulation of HER3 by Akt. We have now further characterized HER2-HER3 signaling activity and have shown that the compensatory buffering prevents apoptotic tumor cell death from occurring as a result of the combined loss of mitogen-activated protein kinase (MAPK) and Akt signaling. To overcome the cancer cells' compensatory mechanisms, we coadministered a phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor and a HER2 tyrosine kinase inhibitor (TKI). This treatment strategy proved equivocal because it induced both TKI-sensitizing and TKI-desensitizing effects and robust cross-compensation of MAPK and Akt signaling pathways. Noting that HER2-HER3 activity was completely inhibited by higher, fully inactivating doses of TKI, we then attempted to overcome the cells' compensatory buffering with this higher dose. This treatment crippled all downstream signaling and induced tumor apoptosis. Although such high doses of TKI are toxic in vivo when given continuously, we found that intermittent doses of TKI administered to mice produced sequential cycles of tumor apoptosis and ultimately complete tumor regression in mouse models, with little toxicity. This strategy for inactivation of HER2-HER3 tumorigenic activity is proposed for clinical testing.
Figures



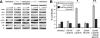


References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177. - PubMed
-
- Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007 May;7:389. - PubMed
-
- Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, Innocent N, Cardiff RD, Schnall MD, Chodosh LA. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002 Dec;2:451. - PubMed
-
- Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, Forster JK, Rubin SD, Stein SH, Burstein HJ. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009 Jun;20:1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

