Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction
- PMID: 20371544
- PMCID: PMC2876883
- DOI: 10.1093/hmg/ddq110
Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction
Abstract
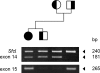
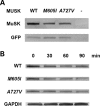
We describe a severe congenital myasthenic syndrome (CMS) caused by two missense mutations in the gene encoding the muscle specific receptor tyrosine kinase (MUSK). The identified MUSK mutations M605I and A727V are both located in the kinase domain of MuSK. Intracellular microelectrode recordings and microscopy studies of the neuromuscular junction conducted in an anconeus muscle biopsy revealed decreased miniature endplate potential amplitudes, reduced endplate size and simplification of secondary synaptic folds, which were consistent with postsynaptic deficit. The study also showed a striking reduction of the endplate potential quantal content, consistent with additional presynaptic failure. Expression studies in MuSK deficient myotubes revealed that A727V, which is located within the catalytic loop of the enzyme, caused severe impairment of agrin-dependent MuSK phosphorylation, aggregation of acetylcholine receptors (AChRs) and interaction of MuSK with Dok-7, an essential intracellular binding protein of MuSK. In contrast, M605I, resulted in only moderate impairment of agrin-dependent MuSK phosphorylation, aggregation of AChRs and interaction of MuSK with Dok-7. There was no impairment of interaction of mutants with either the low-density lipoprotein receptor-related protein, Lrp4 (a co-receptor of agrin) or with the mammalian homolog of the Drosophila tumorous imaginal discs (Tid1). Our findings demonstrate that missense mutations in MUSK can result in a severe form of CMS and indicate that the inability of MuSK mutants to interact with Dok-7, but not with Lrp4 or Tid1, is a major determinant of the pathogenesis of the CMS caused by MUSK mutations.
Figures





References
-
- Hantaï D., Richard P., Koenig J., Eymard B. Congenital myasthenic syndromes. Curr. Opin. Neurol. 2004;17:539–551. doi:10.1097/00019052-200410000-00004. - DOI - PubMed
-
- Beeson D., Webster R., Cossins J., Lashley D., Spearman H., Maxwell S., Slater C.R., Newsom-Davis J., Palace J., Vincent A. Congenital myasthenic syndromes and the formation of the neuromuscular junction. Ann. N. Y. Acad. Sci. 2008;1132:99–103. doi:10.1196/annals.1405.049. - DOI - PubMed
-
- Engel A.G., Shen X.M., Selcen D., Sine S.M. Further observations in congenital myasthenic syndromes. Ann. N. Y. Acad. Sci. 2008;1132:104–113. doi:10.1196/annals.1405.039. - DOI - PMC - PubMed
-
- Chevessier F., Faraut B., Ravel-Chapuis A., Richard P., Gaudon K., Bauché S., Prioleau C., Herbst R., Goillot E., Ioos C., et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum. Mol. Genet. 2004;13:3229–3240. doi:10.1093/hmg/ddh333. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

