Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline
- PMID: 20371621
- PMCID: PMC2909293
- DOI: 10.1096/fj.09-154054
Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline
Abstract
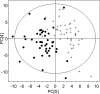
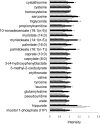
Choline is an essential nutrient, and deficiency causes liver and muscle dysfunction. Common genetic variations alter the risk of developing organ dysfunction when choline deficient, probably by causing metabolic inefficiencies that should be detectable even while ingesting a normal choline-adequate diet. We determined whether metabolomic profiling of plasma at baseline could predict whether humans will develop liver dysfunction when deprived of dietary choline. Fifty-three participants were fed a diet containing 550 mg choline/70 kg/d for 10 d and then fed < 50 mg choline/70 kg/d for up to 42 d. Participants who developed organ dysfunction on this diet were repleted with a choline-adequate diet for > or = 3 d. Plasma samples, obtained at baseline, end of depletion, and end of repletion, were used for targeted and nontargeted metabolomic profiling. Liver fat was assessed using magnetic resonance spectroscopy. Metabolomic profiling and targeted biochemical analyses were highly correlated for the analytes assessed by both procedures. In addition, we report relative concentration changes of other small molecules detected by the nontargeted metabolomic analysis after choline depletion. Finally, we show that metabolomic profiles of participants when they were consuming a control baseline diet could predict whether they would develop liver dysfunction when deprived of dietary choline.
Figures






References
-
- Niculescu M. D., Zeisel S. H. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. - PubMed
-
- Finkelstein J. D. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26:219–225. - PubMed
-
- Kim Y. I., Miller J. W., da Costa K. A., Nadeau M., Smith D., Selhub J., Zeisel S. H., Mason J. B. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J Nutr. 1995;124:2197–2203. - PubMed
-
- Selhub J., Seyoum E., Pomfret E. A., Zeisel S. H. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

