Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells
- PMID: 20371630
- PMCID: PMC2909282
- DOI: 10.1096/fj.09-148007
Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells
Abstract
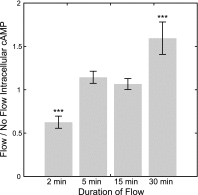
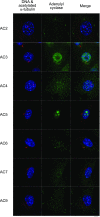
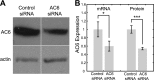
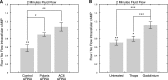
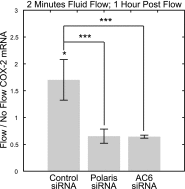
Primary cilia are chemosensing and mechanosensing organelles that regulate remarkably diverse processes in a variety of cells. We previously showed that primary cilia play a role in mediating mechanosensing in bone cells through an unknown mechanism that does not involve extracellular Ca(2+)-dependent intracellular Ca(2+) release, which has been implicated in all other cells that transduce mechanical signals via the cilium. Here, we identify a molecular mechanism linking primary cilia and bone cell mechanotransduction that involves adenylyl cyclase 6 (AC6) and cAMP. Intracellular cAMP was quantified in MLO-Y4 cells exposed to dynamic flow, and AC6 and primary cilia were inhibited using RNA interference. When exposed to flow, cells rapidly (<2 min) and transiently decreased cAMP production in a primary cilium-dependent manner. RT-PCR revealed differential expression of the membrane-bound isoforms of adenylyl cyclase, while immunostaining revealed one, AC6, preferentially localized to the cilium. Further studies showed that decreases in cAMP in response to flow were dependent on AC6 and Gd(3+)-sensitive channels but not intracellular Ca(2+) release and that this response mediated flow-induced COX-2 gene expression. The signaling events identified provide important details of a novel early mechanosensing mechanism in bone and advances our understanding of how signal transduction occurs at the primary cilium.
Figures

Similar articles
-
Adenylyl cyclase 3 regulates osteocyte mechanotransduction and primary cilium.Biochem Biophys Res Commun. 2021 Oct 8;573:145-150. doi: 10.1016/j.bbrc.2021.08.033. Epub 2021 Aug 12. Biochem Biophys Res Commun. 2021. PMID: 34411897 Free PMC article.
-
Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo.FASEB J. 2014 Mar;28(3):1157-65. doi: 10.1096/fj.13-240432. Epub 2013 Nov 25. FASEB J. 2014. PMID: 24277577 Free PMC article.
-
Mesenchymal stem cell mechanotransduction is cAMP dependent and regulated by adenylyl cyclase 6 and the primary cilium.J Cell Sci. 2018 Nov 8;131(21):jcs222737. doi: 10.1242/jcs.222737. J Cell Sci. 2018. PMID: 30301777
-
Osteocytes and Primary Cilia.Curr Osteoporos Rep. 2023 Dec;21(6):719-730. doi: 10.1007/s11914-023-00819-1. Epub 2023 Sep 8. Curr Osteoporos Rep. 2023. PMID: 37682373 Free PMC article. Review.
-
Osteocyte primary cilium and its role in bone mechanotransduction.Ann N Y Acad Sci. 2010 Mar;1192:422-8. doi: 10.1111/j.1749-6632.2009.05243.x. Ann N Y Acad Sci. 2010. PMID: 20392268 Free PMC article. Review.
Cited by
-
The role of osteocytes-specific molecular mechanism in regulation of mechanotransduction - A systematic review.J Orthop Translat. 2021 May 13;29:1-9. doi: 10.1016/j.jot.2021.04.005. eCollection 2021 Jul. J Orthop Translat. 2021. PMID: 34036041 Free PMC article. Review.
-
The interaction of biological factors with mechanical signals in bone adaptation: recent developments.Curr Osteoporos Rep. 2012 Jun;10(2):126-31. doi: 10.1007/s11914-012-0099-y. Curr Osteoporos Rep. 2012. PMID: 22538521 Free PMC article. Review.
-
Primary cilia elongation in response to interleukin-1 mediates the inflammatory response.Cell Mol Life Sci. 2012 Sep;69(17):2967-77. doi: 10.1007/s00018-012-0980-y. Epub 2012 Apr 6. Cell Mol Life Sci. 2012. PMID: 22481441 Free PMC article.
-
The molecular basis of bone mechanotransduction.J Musculoskelet Neuronal Interact. 2016 Sep 7;16(3):221-36. J Musculoskelet Neuronal Interact. 2016. PMID: 27609037 Free PMC article. Review.
-
The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia.J Neurosci. 2011 Apr 13;31(15):5557-61. doi: 10.1523/JNEUROSCI.6561-10.2011. J Neurosci. 2011. PMID: 21490195 Free PMC article.
References
-
- Ingber D. E. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:1–14. - PubMed
-
- Knothe Tate M. L. “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech. 2003;36:1409–1424. - PubMed
-
- Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., Burger E. H. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. - PubMed
-
- Vezeridis P. S., Semeins C. M., Chen Q., Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348:1082–1088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

