Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease
- PMID: 20371660
- PMCID: PMC2902076
- DOI: 10.1210/jc.2009-2622
Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease
Abstract
Context: Nonalcoholic fatty liver disease is associated with risk factors for cardiovascular disease, particularly increased plasma triglyceride (TG) concentrations and insulin resistance. Fenofibrate and extended release nicotinic acid (Niaspan) are used to treat hypertriglyceridemia and can affect fatty acid oxidation and plasma free fatty acid concentrations, which influence intrahepatic triglyceride (IHTG) content and metabolic function.
Objective: The objective of the study was to determine the effects of fenofibrate and nicotinic acid therapy on IHTG content and cardiovascular risk factors. EXPERIMENTAL DESIGN AND MAIN OUTCOME MEASURES: We conducted a randomized, controlled trial to determine the effects of fenofibrate (8 wk, 200 mg/d), Niaspan (16 wk, 2000 mg/d), or placebo (8 wk) on IHTG content, very low-density lipoprotein (VLDL) kinetics, and insulin sensitivity.
Setting and participants: Twenty-seven obese subjects with nonalcoholic fatty liver disease (body mass index 36 +/- 1 kg/m(2), IHTG 23 +/- 2%) were studied at Washington University.
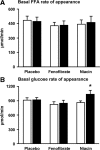
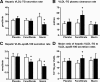
Results: Neither fenofibrate nor Niaspan affected IHTG content, but both decreased plasma TG, VLDL-TG, and VLDL-apolipoprotein B concentrations (P < 0.05). Fenofibrate increased VLDL-TG clearance from plasma (33 to 54 ml/min; P < 0.05) but not VLDL-TG secretion. Niaspan decreased VLDL-TG secretion (27 to 15 micromol/min; P < 0.05) without affecting clearance. Both fenofibrate and Niaspan decreased VLDL-apolipoprotein B secretion (1.6 to 1.2 and 1.3 to 0.9 nmol/min, respectively; P < 0.05). Niaspan reduced hepatic, adipose tissue, and muscle insulin sensitivity (P < 0.05), whereas fenofibrate had no effect on insulin action.
Conclusions: Fenofibrate and Niaspan decrease plasma VLDL-TG concentration without altering IHTG content. However, the mechanism responsible for the change in VLDL-TG concentration is different for each drug; fenofibrate increases plasma VLDL-TG clearance, whereas nicotinic acid decreases VLDL-TG secretion.
Figures
References
-
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N 2001 Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50:1844–1850 - PubMed
-
- Westerbacka J, Cornér A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen AM, Fredriksson J, Yki-Järvinen H 2004 Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 47:1360–1369 - PubMed
-
- Targher G, Arcaro G 2007 Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 191: 235–240 - PubMed
-
- Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H 2005 γ-Glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 112:2130–2137 - PubMed
-
- Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G 2005 Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54:3541–3546 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

