Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma
- PMID: 20371696
- PMCID: PMC2855775
- DOI: 10.1158/1078-0432.CCR-10-0037
Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma
Abstract
Purpose: Molecular biomarkers in blood are promising for assessment of tumor progression and treatment response. We hypothesized that serial monitoring of circulating tumor cells (CTC) with the use of multimarker quantitative real-time reverse transcriptase-PCR assays could be a surrogate predictor of outcome for melanoma patients enrolled in a multicenter phase II clinical trial of biochemotherapy (BCT) combined with maintenance biotherapy (mBT).
Experimental design: Blood specimens were collected from 87 patients before and during induction BCT and mBT for stage IV melanoma. Expression of five melanoma-associated CTC biomarkers (MART-1, GalNAc-T, PAX-3, MAGE-A3, and Mitf) was assessed by quantitative real-time reverse transcriptase-PCR, and correlated with treatment response and disease outcome.
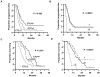
Results: The number of positive CTC biomarkers decreased overall during induction BCT (P < 0.0001). CTC biomarker detection after two cycles of BCT was correlated with treatment response (P = 0.005) and overall survival (P = 0.001): an increase in the number of CTC biomarkers was associated with poor response (P = 0.006) and overall survival (P < 0.0001). Multivariate analyses with the use of a Cox proportional hazards model identified the change in CTC biomarkers after two cycles of BCT as an independent prognostic factor for disease progression (risk ratio, 12.6; 95% confidence interval, 4.78-33.4; P < 0.0001) and overall survival (risk ratio, 6.11; 95% confidence interval, 2.37-15.7; P = 0.0005).
Conclusion: Serial monitoring of CTC during induction BCT may be useful for predicting therapeutic efficacy and disease outcome in patients receiving BCT and mBT for stage IV melanoma.
Figures
References
-
- Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–7. - PubMed
-
- Mellado B, Gutierrez L, Castel T, et al. Prognostic significance of the detection of circulating malignant cells by reverse transcriptase-polymerase chain reaction in long-term clinically disease-free melanoma patients. Clin Cancer Res. 1999;5:1843–8. - PubMed
-
- Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–13. - PubMed
-
- Palmieri G, Strazzullo M, Ascierto PA, Satriano SM, Daponte A, Castello G. Polymerase chain reaction-based detection of circulating melanoma cells as an effective marker of tumor progression. Melanoma Cooperative Group. J Clin Oncol. 1999;17:304–11. - PubMed
-
- Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

