Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38
- PMID: 20371725
- PMCID: PMC4077038
- DOI: 10.1158/1535-7163.MCT-09-0996
Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38
Erratum in
- Mol Cancer Ther. 2011 Apr;10(4):708. Karempelas, Ioannis [corrected to Karampelas, Ioannis]
Abstract
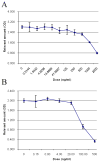
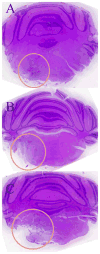
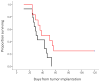
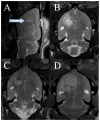
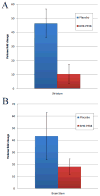
Monoclonal antibodies have the potential to target therapy for high-grade gliomas. Monoclonal antibody 8H9 is specific for membrane protein B7H3 and is reactive with most human high-grade gliomas. We tested the 8H9scFv-PE38 recombinant Pseudomonas immunotoxin in a preclinical model of high-grade glioma. The half maximal inhibitory concentration (IC(50)) of 8H9scFv-PE38 in vitro was determined using glioblastoma cell lines U87 and U251. Maximum tolerated infusion dose of 8H9scFv-PE38 following interstitial infusion to the striatum and pons was defined using athymic rats. Maximum tolerated infusion dose of 8H9scFv-PE38 or PBS control were interstitially delivered to athymic rats xenografted with U87 in the striatum or brain stem. Radiographic response and survivals were measured and compared between treatment groups. The in vitro IC(50) of 8H9scFv-PE38 for U87 was 1,265 ng/mL and, for U251, 91 ng/mL. The maximum tolerated infusion doses of interstitially infused 8H9scFv-PE38 to the striatum and brain stem were 0.75 and 1.8 mug, respectively. For rats harboring intracranial U87 xenografts, infusion of 8H9scFv-PE38 increased mean survival (striatum, 43.4 versus 24.6 days; brain stem, 80.6 versus 45.5 days; n = 28 total) and produced three long-term survivors past 120 days. None of the 14 placebo-treated animals survived >54 days. Tumors also showed volumetric response to infusion of 8H9scFv-PE38 by magnetic resonance imaging. Interstitial infusion of 8H9scFv-PE38 shows potential for the treatment of hemispherical and brain stem glioma. Mol Cancer Ther; 9(4); 1039-46. (c)2010 AACR.
Figures
References
-
- Prados M, Lang FF, Sherman JW, et al. Convection-enhanced delivery (CED) by positive pressure infusion for intra-tumoral and pretumoral administration of IL13-PE38QQR a recombinant tumor-targeted cytotoxin in recurrent malignant glioma. Neuro-oncol. 2002;4(Suppl 1):S78.
-
- Olivi A, Grossman SA, Tatter S, et al. Dose Escalation of Carmustine in Surgically Implanted Polymers in Patients With Recurrent Malignant Glioma: A New Approaches to Brain Tumor Therapy CNS Consortium Trial. J Clin Oncol. 2003;21:1845–49. - PubMed
-
- Kondziolka D, Flickinger JC, Bissonette DJ, Bozik M, Lunsford LD. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery. 1997;41:776–83. discussion 83–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

