Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans
- PMID: 20371805
- PMCID: PMC2857506
- DOI: 10.1523/JNEUROSCI.6065-09.2010
Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans
Abstract
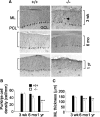
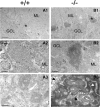
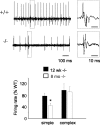
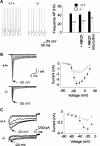
Mutations in SPTBN2, the gene encoding beta-III spectrin, cause spinocerebellar ataxia type 5 in humans (SCA5), a neurodegenerative disorder resulting in loss of motor coordination. How these mutations give rise to progressive ataxia and what the precise role beta-III spectrin plays in normal cerebellar physiology are unknown. We developed a mouse lacking full-length beta-III spectrin and found that homozygous mice reproduced features of SCA5 including gait abnormalities, tremor, deteriorating motor coordination, Purkinje cell loss, and cerebellar atrophy (molecular layer thinning). In vivo analysis reveals an age-related reduction in simple spike firing rate in surviving beta-III(-/-) Purkinje cells, whereas in vitro studies show these neurons to have reduced spontaneous firing, smaller sodium currents, and dysregulation of glutamatergic neurotransmission. Our data suggest an early loss of EAAT4- (protein interactor of beta-III spectrin) and a subsequent loss of GLAST-mediated uptake may play a role in neuronal pathology. These findings implicate a loss of beta-III spectrin function in SCA5 pathogenesis and indicate that there are at least two physiological effects of beta-III spectrin loss that underpin a progressive loss of inhibitory cerebellar output, namely an intrinsic Purkinje cell membrane defect due to reduced sodium currents and alterations in glutamate signaling.
Figures



References
-
- Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37:796–803. - PubMed
-
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. - PubMed
-
- Bürk K, Zühlke C, König IR, Ziegler A, Schwinger E, Globas C, Dichgans J, Hellenbroich Y. Spinocerebellar ataxia type 5: clinical and molecular genetic features of a German kindred. Neurology. 2004;62:327–329. - PubMed
-
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
