Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs
- PMID: 20372105
- PMCID: PMC2889746
- DOI: 10.1038/mt.2010.51
Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs
Abstract
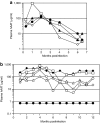
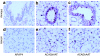
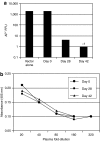
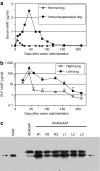
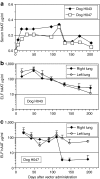
We evaluated the potential of lung-directed gene therapy for alpha1-antitrypsin (AAT) deficiency using an adeno-associated virus type 6 (AAV6) vector containing a human AAT (hAAT) complementary DNA (cDNA) delivered to the lungs of mice and dogs. The results in normal and immune-deficient mice showed that hAAT concentrations were much higher in lung fluid than in plasma, and therapeutic levels were obtained even in normal mice. However, in normal mice an immune response against the vector and/or transgene limited long-term gene expression. An AAV6 vector expressing a marker protein verified that AAV6 vectors efficiently transduced lung cells in dogs. Delivery of AAV6-hAAT resulted in low levels of hAAT in dog serum but therapeutic levels in the lung that persisted for at least 58 days to 4 months in three immunosuppressed dogs. Expression in the serum was not detectable after 45 days in one nonimmune suppressed dog. A lymphoproliferative response to AAV capsid but not to hAAT was detected even after immunosuppression. These results in mice and dogs show the feasibility of expression of therapeutic levels of AAT in the lungs after AAV vector delivery, and advocate for approaches to prevent cellular immune responses to AAV capsid proteins for persistence of gene expression in humans.
Figures

Similar articles
-
Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of alpha-1-antitrypsin using invasive and noninvasive delivery.Mol Ther. 2009 Jan;17(1):81-7. doi: 10.1038/mt.2008.217. Epub 2008 Oct 21. Mol Ther. 2009. PMID: 18941444 Free PMC article.
-
Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method.Hum Gene Ther. 2011 Feb;22(2):155-65. doi: 10.1089/hum.2010.118. Epub 2010 Dec 12. Hum Gene Ther. 2011. PMID: 20812844 Free PMC article.
-
Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results.Hum Gene Ther. 2011 Oct;22(10):1239-47. doi: 10.1089/hum.2011.053. Epub 2011 Aug 24. Hum Gene Ther. 2011. PMID: 21609134 Free PMC article. Clinical Trial.
-
Gene therapy progress and prospects: alpha-1 antitrypsin.Gene Ther. 2003 Jan;10(2):95-9. doi: 10.1038/sj.gt.3301947. Gene Ther. 2003. PMID: 12571637 Review.
-
Advances in Alpha-1 Antitrypsin Gene Therapy.Am J Respir Cell Mol Biol. 2020 Nov;63(5):560-570. doi: 10.1165/rcmb.2020-0159PS. Am J Respir Cell Mol Biol. 2020. PMID: 32668173 Free PMC article. Review.
Cited by
-
Gene Therapy for Alpha-1 Antitrypsin Deficiency Lung Disease.Ann Am Thorac Soc. 2016 Aug;13 Suppl 4(Suppl 4):S352-69. doi: 10.1513/AnnalsATS.201506-344KV. Ann Am Thorac Soc. 2016. PMID: 27564673 Free PMC article. Review.
-
Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer.Mol Ther. 2011 Jun;19(6):1070-8. doi: 10.1038/mt.2011.22. Epub 2011 Mar 1. Mol Ther. 2011. PMID: 21364538 Free PMC article.
-
Analyzing cellular immunity to AAV in a canine model using ELISPOT assay.Methods Mol Biol. 2012;792:65-74. doi: 10.1007/978-1-61779-325-7_5. Methods Mol Biol. 2012. PMID: 21956501 Free PMC article.
-
Capsid-expressing DNA in AAV vectors and its elimination by use of an oversize capsid gene for vector production.Gene Ther. 2011 Apr;18(4):411-7. doi: 10.1038/gt.2010.167. Epub 2010 Dec 16. Gene Ther. 2011. PMID: 21160534 Free PMC article.
-
Robust Lentiviral Gene Delivery But Limited Transduction Capacity of Commonly Used Adeno-Associated Viral Serotypes in Xenotransplanted Human Skin.Hum Gene Ther Methods. 2015 Aug;26(4):123-33. doi: 10.1089/hgtb.2014.135. Hum Gene Ther Methods. 2015. PMID: 26204415 Free PMC article.
References
-
- Stoller JK., and , Aboussouan LS. Myths and misconceptions about α1-antitrypsin deficiency. Arch Intern Med. 2009;169:546–550. - PubMed
-
- Richmond RJ., and , Zellner KM. α1-antitrypsin deficiency: incidence and implications. Dimens Crit Care Nurs. 2005;24:255–60. - PubMed
-
- Abusriwil H., and , Stockley RA. -1-antitrypsin replacement therapy: current status. Curr Opin Pulm Med. 2006;12:125–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

