Intravital microscopy: a novel tool to study cell biology in living animals
- PMID: 20372919
- PMCID: PMC3027219
- DOI: 10.1007/s00418-010-0692-z
Intravital microscopy: a novel tool to study cell biology in living animals
Abstract
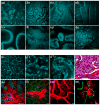
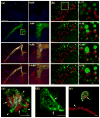
Intravital microscopy encompasses various optical microscopy techniques aimed at visualizing biological processes in live animals. In the last decade, the development of non-linear optical microscopy resulted in an enormous increase of in vivo studies, which have addressed key biological questions in fields such as neurobiology, immunology and tumor biology. Recently, few studies have shown that subcellular processes can be imaged dynamically in the live animal at a resolution comparable to that achieved in cell cultures, providing new opportunities to study cell biology under physiological conditions. The overall aim of this review is to give the reader a general idea of the potential applications of intravital microscopy with a particular emphasis on subcellular imaging. An overview of some of the most exciting studies in this field will be presented using resolution as a main organizing criterion. Indeed, first we will focus on those studies in which organs were imaged at the tissue level, then on those focusing on single cells imaging, and finally on those imaging subcellular organelles and structures.
Figures

References
-
- Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. - PubMed
-
- Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20:54–62. - PubMed
-
- Ashworth SL, Tanner GA. Fluorescent labeling of renal cells in vivo. Nephron Physiol. 2006;103:p91–96. - PubMed
-
- Bestvater F, Spiess E, Stobrawa G, Hacker M, Feurer T, Porwol T, Berchner-Pfannschmidt U, Wotzlaw C, Acker H. Two-photon fluorescence absorption and emission spectra of dyes relevant for cell imaging. J Microsc. 2002;208:108–115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

