High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence
- PMID: 20373327
- PMCID: PMC3050506
- DOI: 10.1002/dev.20444
High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence
Abstract
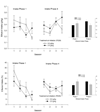
Adolescent initiation of ethanol consumption is associated with subsequent heightened probability of ethanol use disorders. The present study examined the relationship between motivational sensitivity to ethanol initiation in adolescent rats and later ethanol intake. Experiment 1 determined that ethanol induces locomotor activation shortly after administration but not if tested at a later post-administration interval. In Experiment 2, adolescent rats were assessed for ethanol-induced locomotor activation on postnatal Day 28. These animals were then evaluated for ethanol-mediated conditioned taste aversion and underwent a 16-day-long ethanol intake protocol. Ethanol-mediated aversive effects were unrelated to ethanol locomotor stimulation or subsequent ethanol consumption patterns. Ethanol intake during late adolescence was greatest in animals initiated to ethanol earliest at postnatal Day 28. Females that were more sensitive to ethanol's locomotor-activating effects showed a transient increase in ethanol self-administration. Blood ethanol concentrations during initiation were not related to ethanol-induced locomotor activation. Adolescent rats appeared sensitive to the locomotor-stimulatory effects of ethanol. Even brief ethanol exposure during adolescence may promote later ethanol intake.
Figures





References
-
- Acevedo MB, Molina JC, Pautassi RM. Ethanol-induced activation in adolescent rats. Paper presented at the 12th Meeting of the Argentinean Association of Behavioral Sciences, Catholic University; August 27–29; Buenos Aires, Argentina. 2009.
-
- Anderson RI, Varlinskaya EI, Spear LP. Isolation stress and ethanol-induced conditioned taste aversion in adolescent and adult male rats. Paper presented at the 41st Annual Meeting of the International Society for Developmental Psychobiology; Nov. 12–15; Washington DC. 2008.
-
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug and Alcohol Dependence. 1995;40:9–15. - PubMed
-
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behavioral Neuroscience. 2009b;123:172–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

