Triptan-induced latent sensitization: a possible basis for medication overuse headache
- PMID: 20373344
- PMCID: PMC5690477
- DOI: 10.1002/ana.21897
Triptan-induced latent sensitization: a possible basis for medication overuse headache
Abstract
Objective: Identification of the neural mechanisms underlying medication overuse headache resulting from triptans.
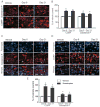
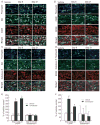
Methods: Triptans were administered systemically to rats by repeated intermittent injections or by continuous infusion over 6 days. Periorbital and hind paw sensory thresholds were measured to detect cutaneous allodynia. Immunofluorescent histochemistry was employed to detect changes in peptidic neurotransmitter expression in identified dural afferents. Enzyme-linked immunoabsorbent assay was used to measure calcitonin gene-related peptide (CGRP) levels in blood.
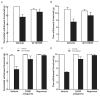
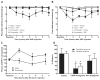
Results: Sustained or repeated administration of triptans to rats elicited time-dependent and reversible cutaneous tactile allodynia that was maintained throughout and transiently after drug delivery. Triptan administration increased labeling for CGRP in identified trigeminal dural afferents that persisted long after discontinuation of triptan exposure. Two weeks after triptan exposure, when sensory thresholds returned to baseline levels, rats showed enhanced cutaneous allodynia and increased CGRP in the blood following challenge with a nitric oxide donor. Triptan treatment thus induces a state of latent sensitization characterized by persistent pronociceptive neural adaptations in dural afferents and enhanced responses to an established trigger of migraine headache in humans.
Interpretation: Triptans represent the treatment of choice for moderate and severe migraine headaches. However, triptan overuse can lead to an increased frequency of migraine headache. Overuse of these medications could induce neural adaptations that result in a state of latent sensitization, which might increase sensitivity to migraine triggers. The latent sensitization could provide a mechanistic basis for the transformation of migraine to medication overuse headache.
Figures


References
-
- Society HCCotIH. The International Classification of Headache Disorders (second edition) Cephalalgia. 2004;24:1–160. - PubMed
-
- Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. - PubMed
-
- Bolay H, Moskowitz MA. The neurobiology of migraine and transformation of headache therapy. In: Waxman S, editor. From neuroscience to neurology: neuroscience, molecular medicine, and the therapeutic transformation of neurology. San Diego, CA: Elsevier; 2004. pp. 107–123.
-
- Bolay H, Moskowitz MA. The emerging importance of cortical spreading depression in migraine headache. Rev Neurol (Paris) 2005;161:655–657. - PubMed
-
- Buzzi MG, Bonamini M, Moskowitz MA. Neurogenic model of migraine. Cephalalgia. 1995;15:277–280. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

