In vitro characterization of the I-neb Adaptive Aerosol Delivery (AAD) system
- PMID: 20373905
- PMCID: PMC3116628
- DOI: 10.1089/jamp.2009.0792
In vitro characterization of the I-neb Adaptive Aerosol Delivery (AAD) system
Abstract
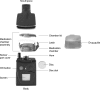
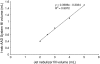
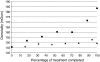
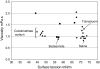
The in vitro characterization of device-related parameters such as the rate of aerosol output, total aerosol output, particle size, and fine particle fraction, is essential when assessing the potential performance of a nebulizer or making comparisons with other nebulizers as they are indicative of potential clinical performance. This article reviews a number of in vitro studies designed to characterize the I-neb Adaptive Aerosol Delivery (AAD) System in terms of drug delivery (particle size, residual, reproducibility, precise dose delivery, dose equivalence), in terms of drug-related performance (osmolality, surface tension, viscosity), and in terms of nebulizer orientation during operation. The results of the in vitro tests of drug delivery indicate that the I-neb AAD System is suitable for delivery of aqueous solutions by nebulization. The evaluation of equivalent doses between the I-neb AAD System (metered dose) and a conventional jet nebulizer (delivered dose), demonstrates that the amount of drug required to deliver the same dose is up to five times less with the I-neb AAD System due to the low residual and controlled drug delivery. The lack of change in osmolality during nebulization might be of importance as it presents an opportunity for delivery of drugs to patients with hyperreactive airways, or where a specific tonicity of the formulation is required. The physicochemical characteristics (surface tension, viscosity) of a number of drugs delivered with the I-neb AAD System highlights some of the demands created by existing and new drug formulations. Finally, the study of the impact of nebulizer orientation shows how important it is to also consider how the nebulizer will actually be physically used by the patient rather than solely under standard conditions used within the laboratory.
Figures
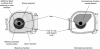

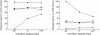
References
-
- Fiel SB. History and evolution of aerosolized therapeutics: overview and introduction. Chest. 2001;120(Suppl):87S–88S. - PubMed
-
- Dolovich MB. Ahrens RC. Hess DR. Anderson P. Dhand R. Rau JL. Smaldone GC. Guyatt G. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–371. - PubMed
-
- Geller DE. Comparing clinical features of the nebulizer, metered-dose inhalers, and dry powder inhaler. Respir Care. 2005;50:1313–1321. - PubMed
-
- Rau JL. Design principles of liquid nebulization devices currently in use. Respir Care. 2002;47:1257–1275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

