Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics
- PMID: 20373994
- PMCID: PMC2883522
- DOI: 10.1089/vim.2009.0088
Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics
Abstract
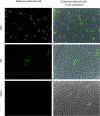
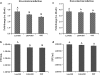
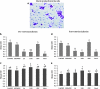
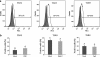
Previous studies of epithelial immune responses to rotavirus infection have been conducted in transformed cell lines. In this study, we evaluated a non-transformed porcine jejunum epithelial cell line (IPEC-J2) as an in-vitro model of rotavirus infection and probiotic treatment. Cell-culture-adapted porcine rotavirus (PRV) OSU strain, or human rotavirus (HRV) Wa strain, along with Lactobacillus acidophilus (LA) or Lactobacillus rhamnosus GG (LGG) were used to inoculate IPEC-J2 cells. LA or LGG treatment was applied pre- or post-rotavirus infection. We demonstrated that IPEC-J2 cells were productively infected by PRV. LA or LGG treatment of the cells did not reduce virus replication. PRV infection increased MUC3 mucin secretion. LGG treatment post-rotavirus infection reduced the mucin secretion response induced by PRV; LGG alone increased the production of membrane-associated MUC3 mucin. LA treatment prior to rotavirus infection significantly increased PRV replication and the IL-6 response to PRV infection, which is consistent with the adjuvant effect of LA. LGG treatment post-rotavirus infection downregulated the IL-6 response, confirming the anti-inflammatory effect of LGG. IPEC-J2 cells expressed toll-like receptor (TLR) 2, TLR3, and TLR9 constitutively. TLR2 expression was upregulated by LGG and peptidoglycan, corresponding to the decreased IL-6 response, indicating that the protective effect of LGG is associated with upregulation of TLR2 expression on intestinal epithelial cells. The IPEC-J2 cell model of PRV infection is a completely homologous system. It is a valuable model for studying the interactions among rotavirus-host-probiotics, and the mechanisms behind the immunomodulating effect of probiotic bacteria on innate immune responses.
Figures
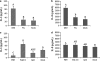
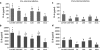
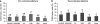
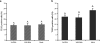
Comment in
-
The quality of papers in Viral Immunology continues to rise.Viral Immunol. 2010 Apr;23(2):125-6. doi: 10.1089/vim.2010.Editorial23.2. Viral Immunol. 2010. PMID: 20373992 No abstract available.
References
-
- Arce C. Ramirez-Boo M. Lucena C. Garrido JJ. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2, IPI-2I) in response to LPS from Salmonella typhimurium. Comp Immunol Microbiol Infect Dis. doi: 10.1016/j.cimid.2008.1008.1003. - PubMed
-
- Azim T. Zaki MH. Podder G, et al. Rotavirus-specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J Med Virol. 2003;69:286–295. - PubMed
-
- Borchers AT. Selmi C. Meyers FJ. Keen CL. Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

