Evaluation of a rapamycin-regulated serotype 2 adeno-associated viral vector in macaque parotid glands
- PMID: 20374510
- PMCID: PMC2852890
- DOI: 10.1111/j.1601-0825.2009.01631.x
Evaluation of a rapamycin-regulated serotype 2 adeno-associated viral vector in macaque parotid glands
Abstract
Objectives: Salivary glands are useful target organs for local and systemic gene therapeutics. For such applications, the regulation of transgene expression is important. Previous studies by us in murine submandibular glands showed that a rapamycin transcriptional regulation system in a single serotype 2, adeno-associated viral (AAV2) vector was effective for this purpose. This study evaluated if such a vector was similarly useful in rhesus macaque parotid glands.
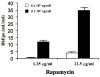
Methods: A recombinant AAV2 vector (AAV-TF-RhEpo-2.3w), encoding rhesus erythropoietin (RhEpo) and a rapamycin-inducible promoter, was constructed. The vector was administered to macaques at either of two doses [1.5 x 10(11) (low dose) or 1.5 x 10(12) (high dose) vector genomes] via cannulation of Stensen's duct. Animals were followed up for 12-14 weeks and treated at intervals with rapamycin (0.1 or 0.5 mg kg(-1)) to induce gene expression. Serum chemistry, hematology, and RhEpo levels were measured at interval.
Results: AAV-TF-RhEpo-2.3w administration led to low levels of rapamycin-inducible RhEpo expression in the serum of most macaques. In five animals, no significant changes were seen in serum chemistry and hematology values over the study. One macaque, however, developed pneumonia, became anemic and subsequently required euthanasia. After the onset of anemia, a single administration of rapamycin led to significant RhEpo production in this animal.
Conclusion: Administration of AAV-TF-RhEpo-2.3w to macaque parotid glands was generally safe, but led only to low levels of serum RhEpo in healthy animals following rapamycin treatment.
Figures


Similar articles
-
AAV5-mediated gene transfer to the parotid glands of non-human primates.Gene Ther. 2010 Jan;17(1):50-60. doi: 10.1038/gt.2009.123. Epub 2009 Sep 17. Gene Ther. 2010. PMID: 19759566 Free PMC article.
-
Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands.Gene Ther. 2006 Jan;13(2):187-90. doi: 10.1038/sj.gt.3302647. Gene Ther. 2006. PMID: 16177817
-
Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates.Hum Gene Ther. 2007 Feb;18(2):142-50. doi: 10.1089/hum.2006.154. Hum Gene Ther. 2007. PMID: 17328682
-
Long-term transduction of miniature pig parotid glands using serotype 2 adeno-associated viral vectors.J Gene Med. 2009 Jun;11(6):506-14. doi: 10.1002/jgm.1319. J Gene Med. 2009. PMID: 19326368 Free PMC article.
-
Assessment of the safety and biodistribution of a regulated AAV2 gene transfer vector after delivery to murine submandibular glands.Toxicol Sci. 2011 Sep;123(1):247-55. doi: 10.1093/toxsci/kfr144. Epub 2011 May 30. Toxicol Sci. 2011. PMID: 21625005 Free PMC article.
Cited by
-
Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease modifying effect in ALS mice.Mol Ther. 2015 Jan;23(1):53-62. doi: 10.1038/mt.2014.180. Epub 2014 Sep 17. Mol Ther. 2015. PMID: 25228069 Free PMC article.
References
-
- Baum BJ, Berkman ME, Marmary Y, et al. Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther. 1999;10:2789–2797. - PubMed
-
- Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10:585–590. - PubMed
-
- Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. - PubMed
-
- Braddon VR, Chiorini JA, Wang S, et al. Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther. 1998;9:2777–2785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

