The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy
- PMID: 20374622
- PMCID: PMC2877678
- DOI: 10.1186/1741-7007-8-33
The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy
Abstract
Background: Neoplastic overgrowth depends on the cooperation of several mutations ultimately leading to major rearrangements in cellular behaviour. Precancerous cells are often removed by cell death from normal tissues in the early steps of the tumourigenic process, but the molecules responsible for such a fundamental safeguard process remain in part elusive. With the aim to investigate the molecular crosstalk occurring between precancerous and normal cells in vivo, we took advantage of the clonal analysis methods that are available in Drosophila for studying the phenotypes due to lethal giant larvae (lgl) neoplastic mutation induced in different backgrounds and tissues.
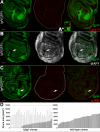
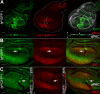
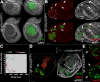
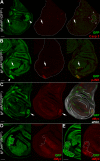
Results: We observed that lgl mutant cells growing in wild-type imaginal wing discs show poor viability and are eliminated by Jun N-terminal Kinase (JNK)-dependent cell death. Furthermore, they express very low levels of dMyc oncoprotein compared with those found in the surrounding normal tissue. Evidence that this is a cause of lgl mutant cells elimination was obtained by increasing dMyc levels in lgl mutant clones: their overgrowth potential was indeed re-established, with mutant cells overwhelming the neighbouring tissue and forming tumourous masses displaying several cancer hallmarks. Moreover, when lgl mutant clones were induced in backgrounds of slow-dividing cells, they upregulated dMyc, lost apical-basal cell polarity and were able to overgrow. Those phenotypes were abolished by reducing dMyc levels in the mutant clones, thereby confirming its key role in lgl-induced tumourigenesis. Furthermore, we show that the eiger-dependent Intrinsic Tumour Suppressor pathway plays only a minor role in eliminating lgl mutant cells in the wing pouch; lgl-/- clonal death in this region is instead driven mainly by dMyc-induced Cell Competition.
Conclusions: Our results provide the first evidence that dMyc oncoprotein is required in lgl tumour suppressor mutant tissue to promote invasive overgrowth in larval and adult epithelial tissues. Moreover, we show that dMyc abundance inside versus outside the mutant clones plays a key role in driving neoplastic overgrowth.
Figures
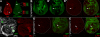
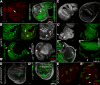
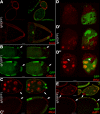
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

