Transcriptional control of brown fat development
- PMID: 20374957
- PMCID: PMC2857670
- DOI: 10.1016/j.cmet.2010.03.005
Transcriptional control of brown fat development
Abstract
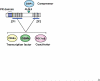
Deconvoluting the natural pathway of BAT development has defined key molecular events, which enables researchers to manipulate the amount or activity of brown fat. We review recent advances on the transcriptional regulation of BAT development and discuss the emerging questions.
2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. - PubMed
-
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. - PubMed
-
- Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell metabolism. 2009;10:324–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

