Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness
- PMID: 20374996
- PMCID: PMC4169124
- DOI: 10.1126/scitranslmed.3000659
Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness
Abstract
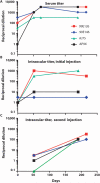
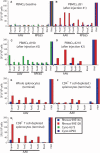
Leber's congenital amaurosis (LCA) is a group of severe inherited retinal degenerations that are symptomatic in infancy and lead to total blindness in adulthood. Recent clinical trials using recombinant adeno-associated virus serotype 2 (rAAV2) successfully reversed blindness in patients with LCA caused by RPE65 mutations after one subretinal injection. However, it was unclear whether treatment of the second eye in the same manner would be safe and efficacious, given the potential for a complicating immune response after the first injection. Here, we evaluated the immunological and functional consequences of readministration of rAAV2-hRPE65v2 to the contralateral eye using large animal models. Neither RPE65-mutant (affected; RPE65(-/-)) nor unaffected animals developed antibodies against the transgene product, but all developed neutralizing antibodies against the AAV2 capsid in sera and intraocular fluid after subretinal injection. Cell-mediated immune responses were benign, with only 1 of 10 animals in the study developing a persistent T cell immune response to AAV2, a response that was mediated by CD4(+) T cells. Sequential bilateral injection caused minimal inflammation and improved visual function in affected animals. Thus, subretinal readministration of rAAV2 in animals is safe and effective, even in the setting of preexisting immunity to the vector, a parameter that has been used to exclude patients from gene therapy trials.
Figures



References
-
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, Cideciyan AV. Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. - PubMed
-
- Lorenz B, Gyürüs P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. - PubMed
-
- Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, Fossarello M, Signorini S, Bertone C, Galantuomo S, Brancati F, Valente EM, Ciccodicola A, Rinaldi E, Auricchio A, Banfi S. Clinical and molecular genetics of Leber’s congenital amaurosis: A multicenter study of Italian patients. Invest. Ophthalmol. Vis. Sci. 2007;48:4284–4290. - PubMed
-
- Perrault I, Rozet JM, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, Dufier JL, Munnich A, Kaplan J. Leber congenital amaurosis. Mol. Genet. Metab. 1999;68:200–208. - PubMed
-
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

