Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza
- PMID: 20375003
- PMCID: PMC2875996
- DOI: 10.1126/scitranslmed.3000678
Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza
Abstract
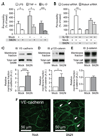
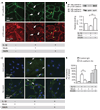
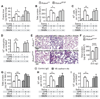
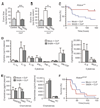
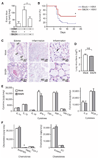
The innate immune system provides a first line of defense against invading pathogens by releasing multiple inflammatory cytokines, such as interleukin-1beta and tumor necrosis factor-alpha, which directly combat the infectious agent and recruit additional immune responses. This exuberant cytokine release paradoxically injures the host by triggering leakage from capillaries, tissue edema, organ failure, and shock. Current medical therapies target individual pathogens with antimicrobial agents or directly either blunt or boost the host's immune system. We explored a third approach: activating with the soluble ligand Slit an endothelium-specific, Robo4-dependent signaling pathway that strengthens the vascular barrier, diminishing deleterious aspects of the host's response to the pathogen-induced cytokine storm. This approach reduced vascular permeability in the lung and other organs and increased survival in animal models of bacterial endotoxin exposure, polymicrobial sepsis, and H5N1 influenza. Thus, enhancing the resilience of the host vascular system to the host's innate immune response may provide a therapeutic strategy for treating multiple infectious agents.
Figures

References
-
- Perdue ML, Swayne DE. Public health risk from avian influenza viruses. Avian Dis. 2005;49:317–327. - PubMed
-
- De Jong JC, Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus: A master of metamorphosis. J. Infect. 2000;40:218–228. - PubMed
-
- Morse SS, Garwin RL, Olsiewski PJ. Public health. Next flu pandemic: What to do until the vaccine arrives? Science. 2006;314:929. - PubMed
-
- Hsieh YC, Wu TZ, Liu DP, Shao PL, Chang LY, Lu CY, Lee CY, Huang FY, Huang LM. Influenza pandemics: Past, present and future. J. Formos. Med. Assoc. 2006;105:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

