Continuous nonradioactive method for screening trypanosomal trans-sialidase activity and its inhibitors
- PMID: 20375068
- PMCID: PMC2895728
- DOI: 10.1093/glycob/cwq056
Continuous nonradioactive method for screening trypanosomal trans-sialidase activity and its inhibitors
Abstract
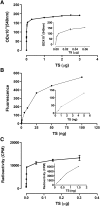
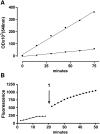
Trypanosoma cruzi, the agent of American trypanosomiasis is unable to synthesize sialic acid (SA). Instead of using the corresponding nucleotide sugar as donor of the monosaccharide, the transfer occurs from alpha-2,3-linked SA in the host sialoglycoconjugates to terminal beta-galactopyranosyl units of the parasite mucins. For that purpose, T. cruzi expresses a glycosylphosphatidylinositol-anchored trans-sialidase (TcTS) that is shed into the milieu, being detected in the blood during the acute phase of the infection. The essential role of TcTS in infection and the absence of a similar activity in mammals make this enzyme an attractive target for the development of alternative chemotherapies. However, there is no effective inhibitor toward this enzyme. In vitro, 3'-sialyllactose (SL) as donor and radioactive lactose as acceptor substrate are widely used to measure TcTS activity. The radioactive sialylated product is then isolated by anion exchange chromatography and measured. Here we describe a new nonradioactive assay using SL or fetuin as donor and benzyl beta-d-Fuc-(1-->6)-alpha-d-GlcNAc (1) as acceptor. Disaccharide 1 was easily synthesized by regioselective glycosylation of benzyl alpha-d-GlcNAc with tetra-O-benzoyl-d-fucose followed by debenzoylation. Compound 1 lacks the hydroxyl group at C-6 of the acceptor galactose and therefore is not a substrate for galactose oxidase. Our method relies on the specific quantification of terminal galactose produced by trans-sialylation from the donor to the 6-deoxy-galactose (D-Fuc) unit of 1 by a spectrophotometric galactose oxidase assay. This method may also discriminate sialidase and trans-sialylation activities by running the assay in the absence of acceptor 1.
Figures



Similar articles
-
Design, synthesis and enzymatic evaluation of 3-O-substituted aryl β-D-galactopyranosides as inhibitors of Trypanosoma cruzi trans-sialidase.Bioorg Med Chem Lett. 2014 Sep 15;24(18):4529-4532. doi: 10.1016/j.bmcl.2014.07.088. Epub 2014 Aug 7. Bioorg Med Chem Lett. 2014. PMID: 25149510
-
Lactose derivatives are inhibitors of Trypanosoma cruzi trans-sialidase activity toward conventional substrates in vitro and in vivo.Glycobiology. 2004 Jul;14(7):659-70. doi: 10.1093/glycob/cwh079. Epub 2004 Apr 7. Glycobiology. 2004. PMID: 15070857
-
The trans-sialidase from Trypanosoma cruzi efficiently transfers alpha-(2-->3)-linked N-glycolylneuraminic acid to terminal beta-galactosyl units.Carbohydr Res. 2007 Nov 26;342(16):2465-9. doi: 10.1016/j.carres.2007.07.018. Epub 2007 Aug 2. Carbohydr Res. 2007. PMID: 17765882
-
Trans-sialidase and mucins of Trypanosoma cruzi: an important interplay for the parasite.Carbohydr Res. 2011 Sep 6;346(12):1389-93. doi: 10.1016/j.carres.2011.04.006. Epub 2011 Apr 8. Carbohydr Res. 2011. PMID: 21645882 Review.
-
Trypanosomal Trans-sialidases: Valuable Synthetic Tools and Targets for Medicinal Chemistry.Top Curr Chem. 2015;367:231-50. doi: 10.1007/128_2012_330. Top Curr Chem. 2015. PMID: 22648866 Review.
Cited by
-
Sialic acid metabolism and sialyltransferases: natural functions and applications.Appl Microbiol Biotechnol. 2012 May;94(4):887-905. doi: 10.1007/s00253-012-4040-1. Epub 2012 Apr 13. Appl Microbiol Biotechnol. 2012. PMID: 22526796 Free PMC article. Review.
-
trans-Sialylation: a strategy used to incorporate sialic acid into oligosaccharides.RSC Chem Biol. 2021 Nov 23;3(2):121-139. doi: 10.1039/d1cb00176k. eCollection 2022 Feb 9. RSC Chem Biol. 2021. PMID: 35360885 Free PMC article. Review.
-
trans-Sialidase neutralizing antibody detection in Trypanosoma cruzi-infected domestic reservoirs.Clin Vaccine Immunol. 2011 Jun;18(6):984-9. doi: 10.1128/CVI.00535-10. Epub 2011 Apr 6. Clin Vaccine Immunol. 2011. PMID: 21471302 Free PMC article.
References
-
- Agusti R, Couto AS, Campetella OE, Frasch ACC, de Lederkremer RM. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology. 1997;7:731–735. - PubMed
-
- Agusti R, Couto AS, Campetella O, Frasch AC, de Lederkremer RM. Structure of the glycosylphosphatidylinositol-anchor of the trans-sialidase from Trypanosoma cruzi metacyclic trypomastigote forms. Mol Biochem Parasitol. 1998;97:123–131. - PubMed
-
- Agusti R, Paris G, Ratier L, Frasch AC, de Lederkremer RM. Lactose derivatives are inhibitors of Trypanosoma cruzi trans-sialidase activity toward conventional substrates in vitro and in vivo. Glycobiology. 2004;14:659–670. - PubMed
-
- Agusti R, Giorgi ME, Mendoza VM, Gallo-Rodriguez C, de Lederkremer RM. Comparative rates of sialylation by recombinant trans-sialidase and inhibitor properties of synthetic oligosaccharides from Trypanosoma cruzi mucins-containing galactofuranose and galactopyranose. Bioorg Med Chem. 2007;15:2611–2616. - PubMed
-
- Alvarez P, Buscaglia CA, Campetella O. Improving protein pharmacokinetics by genetic fusion to simple amino acid sequences. J Biol Chem. 2004;279:3375–3381. - PubMed

