Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling
- PMID: 20375082
- PMCID: PMC2886862
- DOI: 10.1093/toxsci/kfq111
Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling
Abstract
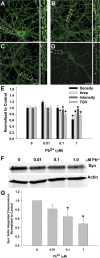
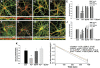
Lead (Pb(2+)) exposure is known to affect presynaptic neurotransmitter release in both in vivo and cell culture models. However, the precise mechanism by which Pb(2+) impairs neurotransmitter release remains unknown. In the current study, we show that Pb(2+) exposure during synaptogenesis in cultured hippocampal neurons produces the loss of synaptophysin (Syn) and synaptobrevin (Syb), two proteins involved in vesicular release. Pb(2+) exposure also increased the number of presynaptic contact sites. However, many of these putative presynaptic contact sites lack Soluble NSF attachment protein receptor complex proteins involved in vesicular exocytosis. Analysis of vesicular release using FM 1-43 dye confirmed that Pb(2+) exposure impaired vesicular release and reduced the number of fast-releasing sites. Because Pb(2+) is a potent N-methyl-D-aspartate receptor (NMDAR) antagonist, we tested the hypothesis that NMDAR inhibition may be producing the presynaptic effects. We show that NMDAR inhibition by aminophosphonovaleric acid mimics the presynaptic effects of Pb(2+) exposure. NMDAR activity has been linked to the signaling of the transsynaptic neurotrophin brain-derived neurotrophic factor (BDNF), and we observed that both the cellular expression of proBDNF and release of BDNF were decreased during the same period of Pb(2+) exposure. Furthermore, exogenous addition of BDNF rescued the presynaptic effects of Pb(2+). We suggest that the presynaptic deficits resulting from Pb(2+) exposure during synaptogenesis are mediated by disruption of NMDAR-dependent BDNF signaling.
Figures






Similar articles
-
Enhanced nitric oxide production during lead (Pb²⁺) exposure recovers protein expression but not presynaptic localization of synaptic proteins in developing hippocampal neurons.Brain Res. 2012 Feb 23;1439:88-95. doi: 10.1016/j.brainres.2011.12.037. Epub 2011 Dec 29. Brain Res. 2012. PMID: 22265330 Free PMC article.
-
Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders.Toxicol Sci. 2012 May;127(1):277-95. doi: 10.1093/toxsci/kfs090. Epub 2012 Feb 17. Toxicol Sci. 2012. PMID: 22345308 Free PMC article.
-
Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition.Neurotoxicology. 2011 Mar;32(2):281-9. doi: 10.1016/j.neuro.2010.12.013. Epub 2010 Dec 28. Neurotoxicology. 2011. PMID: 21192972 Free PMC article.
-
Molecular neurobiology of lead (Pb(2+)): effects on synaptic function.Mol Neurobiol. 2010 Dec;42(3):151-60. doi: 10.1007/s12035-010-8146-0. Epub 2010 Nov 2. Mol Neurobiol. 2010. PMID: 21042954 Free PMC article. Review.
-
Lead neurotoxicity: from exposure to molecular effects.Brain Res Brain Res Rev. 2005 Nov;49(3):529-54. doi: 10.1016/j.brainresrev.2005.02.004. Epub 2005 Mar 31. Brain Res Brain Res Rev. 2005. PMID: 16269318 Review.
Cited by
-
Assessment of neurotransmitter release in human iPSC-derived neuronal/glial cells: a missing in vitro assay for regulatory developmental neurotoxicity testing.Reprod Toxicol. 2023 Apr;117:108358. doi: 10.1016/j.reprotox.2023.108358. Epub 2023 Mar 1. Reprod Toxicol. 2023. PMID: 36863571 Free PMC article.
-
Developmental exposure to Pb2+ induces transgenerational changes to zebrafish brain transcriptome.Chemosphere. 2020 Apr;244:125527. doi: 10.1016/j.chemosphere.2019.125527. Epub 2019 Dec 2. Chemosphere. 2020. PMID: 31816550 Free PMC article.
-
Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio).Toxics. 2024 Apr 11;12(4):282. doi: 10.3390/toxics12040282. Toxics. 2024. PMID: 38668505 Free PMC article.
-
The Effect of Lead Exposure on Autism Development.Int J Mol Sci. 2021 Feb 6;22(4):1637. doi: 10.3390/ijms22041637. Int J Mol Sci. 2021. PMID: 33561959 Free PMC article. Review.
-
Glutamate-Mediated Neural Alterations in Lead Exposure: Mechanisms, Pathways, and Phenotypes.Toxics. 2025 Jun 21;13(7):519. doi: 10.3390/toxics13070519. Toxics. 2025. PMID: 40710964 Free PMC article. Review.
References
-
- Alkondon M, Costa ACS, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990;261:124–130. - PubMed
-
- Bouton CMLS, Frelin LP, Forde CE, Godwin HA, Pevsner J. Synaptotagmin I is a molecular target for lead. J. Neurochem. 2001;76:1724–1735. - PubMed
-
- Braga MFM, Pereira EFR, Albuquerque EX. Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Res. 1999;826:22–34. - PubMed
-
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

